Microfluidic devices integrating microcavity surface-plasmon-resonance sensors: glucose oxidase binding-activity detection
- PMID: 19968248
- PMCID: PMC2824604
- DOI: 10.1021/ac902038d
Microfluidic devices integrating microcavity surface-plasmon-resonance sensors: glucose oxidase binding-activity detection
Abstract
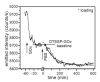
We have developed miniature (approximately 1 microm diameter) microcavity surface-plasmon-resonance sensors (MSPRS), integrated them with microfluidics, and tested their sensitivity to refractive-index changes. We tested their biosensing capability by distinguishing the interaction of glucose oxidase (M(r) 160 kDa) with its natural substrate (beta-D-glucose, M(r) 180 Da) from its interactions with nonspecific substrates (L-glucose, D-mannose, and 2-deoxy-D-glucose). We ran the identical protocol we had used with the MSPRS on a Biacore 3000 instrument using their bare gold chip. Only the MSPRS was able to detect beta-D-glucose binding to glucose oxidase. Each MSPRS can detect the binding to its surface of fewer than 35,000 glucose oxidase molecules (representing 9.6 fg or 60 zmol of protein), about 10(6) times fewer than classical surface-plasmon-resonance biosensors.
Figures




References
-
- Zhong W, Sternberg PW. Science. 2006;311:1481–1484. - PubMed
-
- Formstecher E, Aresta S, Collura V, Hamburger A, Meil A, Trehin A, Reverdy C, Betin V, Maire S, Brun C, Jacq B, Arpin M, Bellaiche Y, Bellusci S, Benaroch P, Bornens M, Chanet R, Chavrier P, Delattre O, Doye V, Fehn R, Faye G, Galli T, Girault J-A, Goud B, deGunzburg J, Johannes L, Junier M-P, Mirouse V, Mukherjee A, Papadopoulo D, Perez F, Plessis A, Rosse C, Saule S, Stoppa-Lyonnet D, Vincent A, White M, Pegrain P, Wojcik J, Camonis J, Daviet L. Genome Res. 2005;15:376–384. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

