Oct-4 controls cell-cycle progression of embryonic stem cells
- PMID: 19968627
- PMCID: PMC2825734
- DOI: 10.1042/BJ20091439
Oct-4 controls cell-cycle progression of embryonic stem cells
Abstract
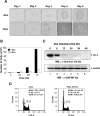
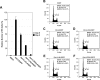
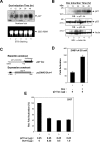
Mouse and human ES (embryonic stem) cells display unusual proliferative properties and can produce pluripotent stem cells indefinitely. Both processes might be important for maintaining the 'stemness' of ES cells; however, little is known about how the cell-cycle fate is regulated in ES cells. Oct-4, a master switch of pluripotency, plays an important role in maintaining the pluripotent state of ES cells and may prevent the expression of genes activated during differentiation. Using ZHBTc4 ES cells, we have investigated the effect of Oct-4 on ES cell-cycle control, and we found that Oct-4 down-regulation in ES cells inhibits proliferation by blocking cell-cycle progression in G0/G1. Deletion analysis of the functional domains of Oct-4 indicates that the overall integrity of the Oct-4 functional domains is important for the stimulation of S-phase entry. We also show in the present study that the p21 gene is a target for Oct-4 repression. Furthermore, p21 protein levels were repressed by Oct-4 and were induced by the down-regulation of Oct-4 in ZHBTc4 ES cells. Therefore the down-regulation of p21 by Oct-4 may contribute to the maintenance of ES cell proliferation.
Figures


Similar articles
-
The self-renewal function of Oct-4 can be replaced by the EWS-Oct-4 fusion protein in embryonic stem cells.Cell Mol Life Sci. 2025 Apr 18;82(1):166. doi: 10.1007/s00018-025-05701-0. Cell Mol Life Sci. 2025. PMID: 40251420 Free PMC article.
-
The reprogramming factor nuclear receptor subfamily 5, group A, member 2 cannot replace octamer-binding transcription factor 4 function in the self-renewal of embryonic stem cells.FEBS J. 2014 Feb;281(4):1029-45. doi: 10.1111/febs.12665. Epub 2013 Dec 16. FEBS J. 2014. PMID: 24341592
-
The human OCT-4 isoforms differ in their ability to confer self-renewal.J Biol Chem. 2006 Nov 3;281(44):33554-65. doi: 10.1074/jbc.M603937200. Epub 2006 Sep 1. J Biol Chem. 2006. PMID: 16951404
-
Transcriptional regulation of the Oct4 gene, a master gene for pluripotency.Histol Histopathol. 2010 Mar;25(3):405-12. doi: 10.14670/HH-25.405. Histol Histopathol. 2010. PMID: 20054811 Free PMC article. Review.
-
Cell cycle and pluripotency: Convergence on octamer‑binding transcription factor 4 (Review).Mol Med Rep. 2017 Nov;16(5):6459-6466. doi: 10.3892/mmr.2017.7489. Epub 2017 Sep 13. Mol Med Rep. 2017. PMID: 28901500 Free PMC article. Review.
Cited by
-
The polycomb group protein L3mbtl2 assembles an atypical PRC1-family complex that is essential in pluripotent stem cells and early development.Cell Stem Cell. 2012 Sep 7;11(3):319-32. doi: 10.1016/j.stem.2012.06.002. Epub 2012 Jul 5. Cell Stem Cell. 2012. PMID: 22770845 Free PMC article.
-
The abbreviated pluripotent cell cycle.J Cell Physiol. 2013 Jan;228(1):9-20. doi: 10.1002/jcp.24104. J Cell Physiol. 2013. PMID: 22552993 Free PMC article. Review.
-
The cell cycle in stem cell proliferation, pluripotency and differentiation.Nat Cell Biol. 2019 Sep;21(9):1060-1067. doi: 10.1038/s41556-019-0384-4. Epub 2019 Sep 2. Nat Cell Biol. 2019. PMID: 31481793 Free PMC article. Review.
-
Molecular Mechanism of Hippo-YAP1/TAZ Pathway in Heart Development, Disease, and Regeneration.Front Physiol. 2020 Apr 23;11:389. doi: 10.3389/fphys.2020.00389. eCollection 2020. Front Physiol. 2020. PMID: 32390875 Free PMC article. Review.
-
Endometrial stem/progenitor cells: Properties, origins, and functions.Genes Dis. 2022 Aug 31;10(3):931-947. doi: 10.1016/j.gendis.2022.08.009. eCollection 2023 May. Genes Dis. 2022. PMID: 37396532 Free PMC article. Review.
References
-
- Niwa H. Molecular mechanism to maintain stem cell renewal of ES cells. Cell Struct. Funct. 2001;26:137–148. - PubMed
-
- Okamoto K., Okazawa H., Okuda A., Sakai M., Muramatsu M., Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990;60:461–472. - PubMed
-
- Rosner M. H., Vigano M. A., Ozato K., Timmons P. M., Poirier F., Rigby P. W., Staudt L. M. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345:686–692. - PubMed
-
- Goto T., Adjaye J., Rodeck C. H., Monk M. Identification of genes expressed in human primordial germ cells at the time of entry of the female germ line into meiosis. Mol. Hum. Reprod. 1999;5:851–860. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

