Proteolytic activation and function of the cytokine Spätzle in the innate immune response of a lepidopteran insect, Manduca sexta
- PMID: 19968713
- PMCID: PMC2805777
- DOI: 10.1111/j.1742-4658.2009.07465.x
Proteolytic activation and function of the cytokine Spätzle in the innate immune response of a lepidopteran insect, Manduca sexta
Abstract
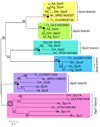
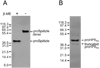
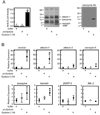
The innate immune response of insects includes induced expression of genes encoding a variety of antimicrobial peptides. The signaling pathways that stimulate this gene expression have been well characterized by genetic analysis in Drosophila melanogaster, but are not well understood in most other insect species. One such pathway involves proteolytic activation of a cytokine called Spätzle, which functions in dorsal-ventral patterning in early embryonic development and in the antimicrobial immune response in larvae and adults. We have investigated the function of Spätzle in a lepidopteran insect, Manduca sexta, in which hemolymph proteinases activated during immune responses have been characterized biochemically. Two cDNA isoforms for M. sexta Spätzle-1 differ because of alternative splicing, resulting in a 10 amino acid residue insertion in the pro-region of proSpätzle-1B that is not present in proSpätzle-1A. The proSpätzle-1A cDNA encodes a 32.7 kDa polypeptide that is 23% and 44% identical to D. melanogaster and Bombyx mori Spätzle-1, respectively. Recombinant proSpätzle-1A was a disulfide-linked homodimer. M. sexta hemolymph proteinase 8 cleaved proSpätzle-1A to release Spätzle-C108, a dimer of the C-terminal 108 residue cystine-knot domain. Injection of Spätzle-C108, but not proSpätzle-1A, into larvae stimulated expression of several antimicrobial peptides and proteins, including attacin-1, cecropin-6, moricin, lysozyme, and the immunoglobulin domain protein hemolin, but did not significantly affect the expression of two bacteria-inducible pattern recognition proteins, immulectin-2 and beta-1,3-glucan recognition protein-2. The results of this and other recent studies support a model for a pathway in which the clip-domain proteinase pro-hemolymph proteinase 6 becomes activated in plasma upon exposure to Gram-negative or Gram-positive bacteria or to beta-1,3-glucan. Hemolymph proteinase 6 then activates pro-hemolymph proteinase 8, which in turn activates Spätzle-1. The resulting Spätzle-C108 dimer is likely to function as a ligand to activate a Toll pathway in M. sexta as a response to a wide variety of microbial challenges, stimulating a broad response to infection. Structured digital abstract * MINT-7295125: Spätzle 1A (uniprotkb:C8BMD1) and Spätzle 1A (uniprotkb:C8BMD1) bind (MI:0407) by comigration in gel electrophoresis (MI:0807).
Figures





Similar articles
-
A Toll-Spätzle pathway in the tobacco hornworm, Manduca sexta.Insect Biochem Mol Biol. 2012 Jul;42(7):514-24. doi: 10.1016/j.ibmb.2012.03.009. Epub 2012 Apr 6. Insect Biochem Mol Biol. 2012. PMID: 22516181 Free PMC article.
-
Serpin-1 splicing isoform J inhibits the proSpätzle-activating proteinase HP8 to regulate expression of antimicrobial hemolymph proteins in Manduca sexta.Dev Comp Immunol. 2011 Jan;35(1):135-41. doi: 10.1016/j.dci.2010.09.004. Epub 2010 Sep 22. Dev Comp Immunol. 2011. PMID: 20851714 Free PMC article.
-
Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways.J Biol Chem. 2009 Jul 17;284(29):19716-26. doi: 10.1074/jbc.M109.007112. Epub 2009 Jun 1. J Biol Chem. 2009. PMID: 19487692 Free PMC article.
-
Innate immune responses of a lepidopteran insect, Manduca sexta.Immunol Rev. 2004 Apr;198:97-105. doi: 10.1111/j.0105-2896.2004.0121.x. Immunol Rev. 2004. PMID: 15199957 Review.
-
Pattern recognition proteins in Manduca sexta plasma.Insect Biochem Mol Biol. 2002 Oct;32(10):1287-93. doi: 10.1016/s0965-1748(02)00091-7. Insect Biochem Mol Biol. 2002. PMID: 12225919 Review.
Cited by
-
Molecular Identification and Immunity Functional Characterization of Lmserpin1 in Locusta migratoria manilensis.Insects. 2021 Feb 18;12(2):178. doi: 10.3390/insects12020178. Insects. 2021. PMID: 33670781 Free PMC article.
-
Changes in composition and levels of hemolymph proteins during metamorphosis of Manduca sexta.Insect Biochem Mol Biol. 2020 Dec;127:103489. doi: 10.1016/j.ibmb.2020.103489. Epub 2020 Oct 20. Insect Biochem Mol Biol. 2020. PMID: 33096211 Free PMC article.
-
Identification of plasma proteinase complexes with serpin-3 in Manduca sexta.Insect Biochem Mol Biol. 2012 Dec;42(12):946-55. doi: 10.1016/j.ibmb.2012.09.008. Epub 2012 Oct 9. Insect Biochem Mol Biol. 2012. PMID: 23063421 Free PMC article.
-
Critical Roles of Spätzle5 in Antimicrobial Peptide Production Against Escherichia coli in Tenebrio molitor Malpighian Tubules.Front Immunol. 2021 Dec 16;12:760475. doi: 10.3389/fimmu.2021.760475. eCollection 2021. Front Immunol. 2021. PMID: 34975850 Free PMC article.
-
Hemolymph protease-5 links the melanization and Toll immune pathways in the tobacco hornworm, Manduca sexta.Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23581-23587. doi: 10.1073/pnas.2004761117. Epub 2020 Sep 8. Proc Natl Acad Sci U S A. 2020. PMID: 32900946 Free PMC article.
References
-
- Kanost MR, Gorman MJ. Phenoloxidase in insect immunity. In: Beckage N, editor. Insect Immunology. San Diego: Academic Press/Elsevier; 2008. pp. 69–96.
-
- Cerenius L, Lee BL, Soderhall K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29:263–271. - PubMed
-
- Yu X, Prakash O, Kanost MR. Insect ENF family peptides with diverse biological activities display similar core structures in solution. Medical Chem. Res. 2001;10:493–501.
-
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat.Rev.Immunol. 2007;7:862–874. - PubMed
-
- Gillespie JP, Kanost MR, Trenczek T. Biological mediators of insect immunity. Annu. Rev. Entomol. 1997;42:611–643. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

