The coagulation factor Xa/protease activated receptor-2 axis in the progression of liver fibrosis: a multifaceted paradigm
- PMID: 19968736
- PMCID: PMC3837617
- DOI: 10.1111/j.1582-4934.2009.00980.x
The coagulation factor Xa/protease activated receptor-2 axis in the progression of liver fibrosis: a multifaceted paradigm
Abstract
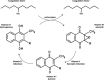
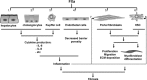
Hepatic fibrosis is a common response to virtually all forms of chronic liver injury independent of the etiologic agent. Despite the relatively large population of patients suffering from hepatic fibrosis and cirrhosis, no efficient and well-tolerated drugs are available for the treatment of this disorder. The lack of efficient treatment options is at least partly because the underlying cellular mechanisms leading to hepatic fibrosis are only partly understood. It is thus of pivotal importance to better understand the cellular processes contributing to the progression of hepatic fibrosis. Interestingly in this perspective, a common feature of fibrotic disease of various organs is the activation of the coagulation cascade and hepatic fibrosis is also accompanied by a local hypercoagulable state. Activated blood coagulation factors directly target liver cells by activating protease-activated receptors (PAR) thereby inducing a plethora of cellular responses like (among others) proliferation, migration and extracellular matrix production. Coagulation factor driven PAR activation thus establishes a potential link between activation of the coagulation cascade and the progression of fibrosis. The current review focuses on blood coagulation factor Xa and summarizes the variety of cellular functions induced by factor Xa-driven PAR-2 activation and the subsequent consequences for tissue repair and hepatic fibrosis.
Figures

References
-
- Schluger LK, Sheiner PA, Thung SN, et al. Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology. 1996;23:971–6. - PubMed
-
- Bonnard P, Lescure FX, Amiel C, et al. Documented rapid course of hepatic fibrosis between two biopsies in patients coinfected by HIV and HCV despite high CD4 cell count. J Viral Hepat. 2007;14:806–11. - PubMed
-
- Lotersztajn S, Julien B, Teixeira-Clerc F, et al. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol. 2005;45:605–28. - PubMed
-
- Svegliati-Baroni G, De Minicis S, Marzioni M. Hepatic fibrogenesis in response to chronic liver injury: novel insights on the role of cell-to-cell interaction and transition. Liver Int. 2008;28:1052–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

