BACE1 and BACE2 enzymatic activities in Alzheimer's disease
- PMID: 19968762
- PMCID: PMC2819564
- DOI: 10.1111/j.1471-4159.2009.06528.x
BACE1 and BACE2 enzymatic activities in Alzheimer's disease
Abstract
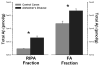
beta-Secretase is the rate limiting enzymatic activity in the production of the amyloid-beta peptide (Abeta) and is thought to be involved in Alzheimer's disease (AD) pathogenesis. Although BACE1 (beta-site APP Cleaving Enzyme 1, EC 3.4.23.46) has received significant attention, the related BACE2 (EC 3.4.23.45) has not. Though BACE2 is also expressed in the brain, its potential role in AD has not been resolved. In this study, we compared the activities of both BACE1 and BACE2, which were isolated from the same samples of frontal cortex from both AD-affected individuals and age-matched controls. BACE1 activity showed a significant positive correlation with the amount of extractable Abeta, and BACE1 protein and activity were significantly increased in AD cases. Unexpectedly, there were substantial total amounts of BACE2 protein and enzymatic activity in the human brain. BACE2 activity did not change significantly in the AD brain, and was not related to Abeta concentration. These data indicate that BACE1 likely accounts for most of the Abeta produced in the human brain, and that BACE2 activity is not a likely contributor. However, as both forms of BACE compete for the same substrate pool, even small changes in BACE2 activity could have consequences for human disease.
Figures




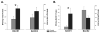
References
-
- Acquati F, Accarino M, Nucci C, Fumagalli P, Jovine L, Ottolenghi S, Taramelli R. The gene encoding DRAP (BACE2), a glycosylated transmembrane protein of the aspartic protease family, maps to the down critical region. FEBS Lett. 2000;468:59–64. - PubMed
-
- Andrau D, Dumanchin-Njock C, Ayral E, et al. BACE1- and BACE2-expressing human cells: characterization of beta-amyloid precursor protein-derived catabolites, design of a novel fluorimetric assay, and identification of new in vitro inhibitors. J Biol Chem. 2003;278:25859–25866. - PubMed
-
- Basi G, Frigon N, Barbour R, Doan T, Gordon G, McConlogue L, Sinha S, Zeller M. Antagonistic effects of beta-site amyloid precursor protein-cleaving enzymes 1 and 2 on beta-amyloid peptide production in cells. J Biol Chem. 2003;278:31512–31520. - PubMed
-
- Bennett BD, Babu-Khan S, Loeloff R, Louis JC, Curran E, Citron M, Vassar R. Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem. 2000;275:20647–20651. - PubMed
-
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

