Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy
- PMID: 19969072
- PMCID: PMC2869084
- DOI: 10.1016/j.freeradbiomed.2009.11.022
Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy
Abstract
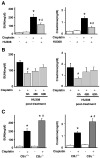
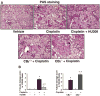
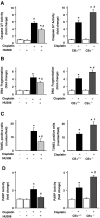
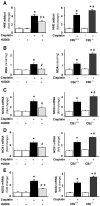
Cisplatin is an important chemotherapeutic agent; however, its nephrotoxicity limits its clinical use. Enhanced inflammatory response and oxidative/nitrosative stress seem to play a key role in the development of cisplatin-induced nephropathy. Activation of cannabinoid-2 (CB(2)) receptors with selective agonists exerts anti-inflammatory and tissue-protective effects in various disease models. We have investigated the role of CB(2) receptors in cisplatin-induced nephrotoxicity using the selective CB(2) receptor agonist HU-308 and CB(2) knockout mice. Cisplatin significantly increased inflammation (leukocyte infiltration, CXCL1/2, MCP-1, TNFalpha, and IL-1beta levels) and expression of adhesion molecule ICAM-1 and superoxide-generating enzymes NOX2, NOX4, and NOX1 and enhanced ROS generation, iNOS expression, nitrotyrosine formation, and apoptotic and poly(ADP-ribose) polymerase-dependent cell death in the kidneys of mice, associated with marked histopathological damage and impaired renal function (elevated serum BUN and creatinine levels) 3 days after the administration of the drug. CB(2) agonist attenuated the cisplatin-induced inflammatory response, oxidative/nitrosative stress, and cell death in the kidney and improved renal function, whereas CB(2) knockouts developed enhanced inflammation and tissue injury. Thus, the endocannabinoid system, through CB(2) receptors, protects against cisplatin-induced kidney damage by attenuating inflammation and oxidative/nitrosative stress, and selective CB(2) agonists may represent a promising novel approach to preventing this devastating complication of chemotherapy.
Published by Elsevier Inc.
Conflict of interest statement
Figures




References
-
- Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8:368–379. - PubMed
-
- Yamate J, Sato K, Ide M, Nakanishi M, Kuwamura M, Sakuma S, Nakatsuji S. Participation of different macrophage populations and myofibroblastic cells in chronically developed renal interstitial fibrosis after cisplatin-induced renal injury in rats. Vet Pathol. 2002;39:322–333. - PubMed
-
- Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther. 2007;322:8–15. - PubMed
-
- Matsushima H, Yonemura K, Ohishi K, Hishida A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med. 1998;131:518–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

