The effects of disruption of genes for peroxiredoxin-2, glutathione peroxidase-1, and catalase on erythrocyte oxidative metabolism
- PMID: 19969073
- PMCID: PMC2818700
- DOI: 10.1016/j.freeradbiomed.2009.11.021
The effects of disruption of genes for peroxiredoxin-2, glutathione peroxidase-1, and catalase on erythrocyte oxidative metabolism
Abstract
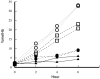
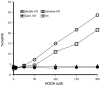
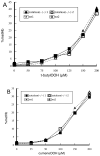
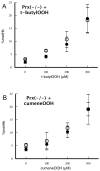
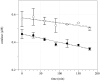
Peroxiredoxin-2 (Prdx2), a potent peroxide reductant, is the third most abundant protein in the erythrocyte and might be expected to play a major role in the cell's oxidative defenses. However, in this study, experiments with erythrocytes from mice with a disrupted Prdx2 gene found that the cells were not more sensitive to exogenous H(2)O(2) or organic peroxides than wild type. Intraerythrocytic H(2)O(2) was increased, however, indicating an important role for Prdx2 in detoxifying endogenously generated H(2)O(2). These results are consistent with proposals that red cell Prdx2 acts stoichiometrically, not catalytically, in reducing peroxides. Additional experiments with mice with disrupted catalase or glutathione peroxidase (Gpx1) genes showed that Gpx1 is the only erythrocyte enzyme that reduces organic peroxides. Catalase(-/-) cells were readily oxidized by exogenous H(2)O(2). Cells lacking both catalase and Gpx1 were more sensitive to exogenous H(2)O(2) than cells lacking only catalase. A kinetic model proposed earlier to rationalize results with Gpx1(-/-) erythrocytes also fits the data with Prdx2(-/-) cells and indicates that although Gpx1 and Prdx2 both participate in removing endogenous H(2)O(2), Prdx2 plays a larger role. Although the rate of H(2)O(2) production in the red cell is quite low, Prdx2-deficient mice are anemic, suggesting an important role in erythropoiesis.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures


References
-
- Hsieh H-S, Jaffé E. The metabolism of methemoglobin in human erythrocytes. In: Surgenor DM, editor. The Red Blood Cell. Academic Press; New York City: 1975. pp. 799–824.
-
- Johnson RM, Goyette G, Jr., Ravindranath Y, Ho YS. Hemoglobin autoxidation and regulation of endogenous H2O2 levels in erythrocytes. Free Radic Biol Med. 2005;39:1407–17. - PubMed
-
- Johnson RM, Goyette G, Jr., Ravindranath Y, Ho YS. Oxidation of glutathione peroxidase-deficient red cells by organic peroxides. Blood. 2002;100:1515–6. - PubMed
-
- Flohé L. Glutathione peroxidase: fact and fiction. Ciba Found Symp. 1978;65:95–122. - PubMed
-
- Low FM, Hampton MB, Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal. 2008;10:1621–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

