Preferential encapsulation and stability of La(3)N cluster in 80 atom cages: experimental synthesis and computational investigation of La(3)N@C(79)N
- PMID: 19995073
- PMCID: PMC2796544
- DOI: 10.1021/ja908370t
Preferential encapsulation and stability of La(3)N cluster in 80 atom cages: experimental synthesis and computational investigation of La(3)N@C(79)N
Abstract
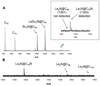
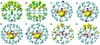
We report the synthesis and electronic stabilization of La(3)N@C(79)N. Unsuccessful efforts to encapsulate bulky La(3)N clusters in small C(80) cages have been attributed to large ionic radii. The preferred species for La(3)N clusters in all-carbon cages is La(3)N@C(96). A surprising finding is the synthesis of La(3)N@C(79)N, a new metallofullerene present in higher abundance than La(3)N@C(96). This reduction in cage size from 96 to 80 atoms reflects the significance and role of electronic effects. To understand the geometric and electronic properties of this first metallic nitride azafullerene (M(3)N@C(79)N, M = La), density functional theory (DFT) investigations were performed on a number of isomers. Results indicate a preferred N-substitution at the 665 junction site on the cage in lieu of a 666 substitution. The relative stabilities of different isomers can be well reproduced by using the minimum distance between the metal atom and the nitrogen atom of the cage (R(N'M)(min)). Long R(N'M)(min) values indicate distant contacts between six atoms that bear significantly large positive charges: the three metal atoms and the three carbon atoms bonded with the nitrogen atom in the cage, which are favored. These results suggest a dominant electronic effect on the stabilities of metalloazafullerenes. Interestingly, spin densities of the 665 substitution isomers of La(3)N@C(79)N are located predominantly in the metal cluster, while spin densities of the 666 substitution isomers are primarily on the cage.
Figures



Similar articles
-
The role of ellipticity on the preferential binding site of Ce and La in C78-D3h--a density functional theory study.Nanoscale. 2010 Jul;2(7):1250-5. doi: 10.1039/c0nr00021c. Epub 2010 May 13. Nanoscale. 2010. PMID: 20648358
-
DFT study on the stabilities of the heterofullerenes Sc3N@C67B, Sc3N@C67N, and Sc3N@C66BN.J Phys Chem A. 2007 Feb 15;111(6):1111-6. doi: 10.1021/jp065097b. Epub 2007 Jan 25. J Phys Chem A. 2007. PMID: 17253661
-
Introducing a novel C50N10 azafullerene with chained nitrogen atoms on a buckyball pole: structure, stability, vibration, and electronic properties.J Mol Model. 2023 Jun 1;29(6):194. doi: 10.1007/s00894-023-05593-6. J Mol Model. 2023. PMID: 37261575
-
Metal nitride cluster fullerenes: their current state and future prospects.Small. 2007 Aug;3(8):1298-320. doi: 10.1002/smll.200700036. Small. 2007. PMID: 17657757 Review.
-
Tri-gadolinium nitride PEGylated-hydroxylated endohedral metallofullerene.2008 Oct 28 [updated 2008 Dec 1]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Oct 28 [updated 2008 Dec 1]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641442 Free Books & Documents. Review.
Cited by
-
Gd2@C79N: isolation, characterization, and monoadduct formation of a very stable heterofullerene with a magnetic spin state of S = 15/2.J Am Chem Soc. 2011 Jun 29;133(25):9741-50. doi: 10.1021/ja202011u. Epub 2011 Jun 6. J Am Chem Soc. 2011. PMID: 21548647 Free PMC article.
-
Inhibition of Aβ42 peptide aggregation by a binuclear ruthenium(II)-platinum(II) complex: Potential for multi-metal organometallics as anti-amyloid agents.ACS Chem Neurosci. 2010 Aug 23;2010(10):691-701. doi: 10.1021/cn100046m. ACS Chem Neurosci. 2010. PMID: 21221412 Free PMC article.
-
(77)Se chemical shift tensor of L-selenocystine: experimental NMR measurements and quantum chemical investigations of structural effects.J Phys Chem B. 2015 Mar 5;119(9):3643-50. doi: 10.1021/jp510857s. Epub 2015 Feb 18. J Phys Chem B. 2015. PMID: 25654666 Free PMC article.
-
Liposome-Templated Green Synthesis of Mesoporous Metal Nanostructures with Universal Composition for Biomedical Application.Small. 2023 Nov;19(46):e2304880. doi: 10.1002/smll.202304880. Epub 2023 Jul 14. Small. 2023. PMID: 37452439 Free PMC article.
-
Insights into the Observed trans-Bond Length Variations upon NO Binding to Ferric and Ferrous Porphyrins with Neutral Axial Ligands.ACS Omega. 2021 Sep 15;6(38):24777-24787. doi: 10.1021/acsomega.1c03610. eCollection 2021 Sep 28. ACS Omega. 2021. PMID: 34604659 Free PMC article.
References
-
- Stevenson S, Rice G, Glass T, Harich K, Cromer F, Jordan MR, Craft J, Hadju E, Bible R, Olmstead MM, Maitra K, Fisher AJ, Balch AL, Dorn HC. Nature. 1999;401:55–57.
-
- Chaur MN, Athans AJ, Echegoyen L. Tetrahedron. 2008;64:11387–11393.
-
- Dunsch L, Yang S. Small. 2007;3:1298–1320. - PubMed
-
- Dunsch L, Yang SF. PCCP. 2007;9:3067–3081. - PubMed
-
- Stevenson S, Fowler PW, Heine T, Duchamp JC, Rice G, Glass T, Harich K, Hajdu E, Bible R, Dorn HC. Nature. 2000;408:427–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

