Structures of beta-amyloid peptide 1-40, 1-42, and 1-55-the 672-726 fragment of APP-in a membrane environment with implications for interactions with gamma-secretase
- PMID: 19995075
- PMCID: PMC2791510
- DOI: 10.1021/ja905457d
Structures of beta-amyloid peptide 1-40, 1-42, and 1-55-the 672-726 fragment of APP-in a membrane environment with implications for interactions with gamma-secretase
Abstract
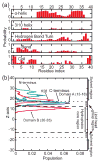
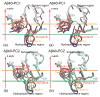
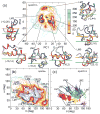
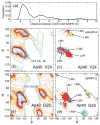
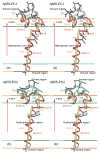
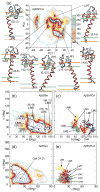
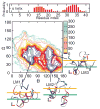
Aggregation of Amyloid beta (Abeta) peptide has been linked to the neurodegenerative Alzheimer's Disease and implicated in other amyloid diseases including cerebral amyloid angiopathy. Abeta peptide is generated by cleavage of the amyloid precursor protein (APP) by transmembrane proteases. It is crucial to determine the structures of beta-amyloid peptides in a membrane to provide a molecular basis for the cleavage mechanism. We report the structures of amyloid beta peptide (Abeta(1-40) and Abeta(1-42)) as well as the 672-726 fragment of APP (referred to as Abeta(1-55)) in a membrane environment determined by replica-exchange molecular dynamics simulation. Abeta(1-40) is found to have two helical domains A (13-22) and B(30-35) and a type I beta-turn at 23-27. The peptide is localized at the interface between membrane and solvent. Substantial fluctuations in domain A are observed. The dominant simulated tertiary structure of Abeta(1-40) is observed to be similar to the simulated Abeta(1-42) structure. However, there are differences observed in the overall conformational ensemble, as characterized by the two-dimensional free energy surfaces. The fragment of APP (Abeta(1-55)) is observed to have a long transmembrane helix. The position of the transmembrane region and ensemble of membrane structures are elucidated. The conformational transition between the transmembrane Abeta(1-55) structure, prior to cleavage, and the Abeta(1-40) structure, following cleavage, is proposed.
Figures



Similar articles
-
Influence of the familial Alzheimer's disease-associated T43I mutation on the transmembrane structure and γ-secretase processing of the C99 peptide.J Biol Chem. 2019 Apr 12;294(15):5854-5866. doi: 10.1074/jbc.RA118.006061. Epub 2019 Feb 12. J Biol Chem. 2019. PMID: 30755484 Free PMC article.
-
Loss of cleavage at β'-site contributes to apparent increase in β-amyloid peptide (Aβ) secretion by β-secretase (BACE1)-glycosylphosphatidylinositol (GPI) processing of amyloid precursor protein.J Biol Chem. 2011 Jul 22;286(29):26166-77. doi: 10.1074/jbc.M111.260471. Epub 2011 Jun 3. J Biol Chem. 2011. PMID: 21642424 Free PMC article.
-
Perturbations of the straight transmembrane α-helical structure of the amyloid precursor protein affect its processing by γ-secretase.J Biol Chem. 2014 Mar 7;289(10):6763-6774. doi: 10.1074/jbc.M113.470781. Epub 2014 Jan 27. J Biol Chem. 2014. PMID: 24469457 Free PMC article.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Gamma-secretase catalyzes sequential cleavages of the AbetaPP transmembrane domain.J Alzheimers Dis. 2009;16(2):211-24. doi: 10.3233/JAD-2009-0957. J Alzheimers Dis. 2009. PMID: 19221413 Free PMC article. Review.
Cited by
-
Structure and Function of Alzheimer's Amyloid βeta Proteins from Monomer to Fibrils: A Mini Review.Protein J. 2019 Aug;38(4):425-434. doi: 10.1007/s10930-019-09854-3. Protein J. 2019. PMID: 31325011 Review.
-
Increased H-Bond Stability Relates to Altered ε-Cleavage Efficiency and Aβ Levels in the I45T Familial Alzheimer's Disease Mutant of APP.Sci Rep. 2019 Mar 29;9(1):5321. doi: 10.1038/s41598-019-41766-1. Sci Rep. 2019. PMID: 30926830 Free PMC article.
-
Competition between homodimerization and cholesterol binding to the C99 domain of the amyloid precursor protein.Biochemistry. 2013 Jul 30;52(30):5051-64. doi: 10.1021/bi400735x. Epub 2013 Jul 18. Biochemistry. 2013. PMID: 23865807 Free PMC article.
-
Silver nanoparticles alter the dimerization of Aβ42 studied by REMD simulations.RSC Adv. 2024 May 8;14(21):15112-15119. doi: 10.1039/d4ra02197e. eCollection 2024 May 2. RSC Adv. 2024. PMID: 38720971 Free PMC article.
-
Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease.Cold Spring Harb Perspect Med. 2012 Jun;2(6):a006262. doi: 10.1101/cshperspect.a006262. Cold Spring Harb Perspect Med. 2012. PMID: 22675658 Free PMC article. Review.
References
-
- Pike C, Walencewicz-Wasserman A, Kosmoski J, Cribbs D, Glabe C, Cotman C. J Neurochem. 1995;64:253–265. - PubMed
-
- Simmons L, May P, Tomaselli K, Rydel R, Fuson K, Brigham E, Wright S, Lieberburg I, Becker G, Brems D. Mol Pharma. 1994;45:373–379. - PubMed
-
- Lynn DG, Meredith SC. J Struct Biol. 2000;130:153–173. - PubMed
-
- Haass C, Selkoe DJ. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

