Activated protein C action in inflammation
- PMID: 19995397
- PMCID: PMC2868910
- DOI: 10.1111/j.1365-2141.2009.08020.x
Activated protein C action in inflammation
Abstract
Activated protein C (APC) is a natural anticoagulant that plays an important role in coagulation homeostasis by inactivating the procoagulation factor Va and VIIIa. In addition to its anticoagulation functions, APC also has cytoprotective effects such as anti-inflammatory, anti-apoptotic, and endothelial barrier protection. Recently, a recombinant form of human APC (rhAPC or drotrecogin alfa activated; known commercially as 'Xigris') was approved by the US Federal Drug Administration for treatment of severe sepsis associated with a high risk of mortality. Sepsis, also known as systemic inflammatory response syndrome (SIRS) resulting from infection, is a serious medical condition in critical care patients. In sepsis, hyperactive and dysregulated inflammatory responses lead to secretion of pro- and anti-inflammatory cytokines, activation and migration of leucocytes, activation of coagulation, inhibition of fibrinolysis, and increased apoptosis. Although initial hypotheses focused on antithrombotic and profibrinolytic functions of APC in sepsis, other agents with more potent anticoagulation functions were not effective in treating severe sepsis. Furthermore, APC therapy is also associated with the risk of severe bleeding in treated patients. Therefore, the cytoprotective effects, rather than the anticoagulant effect of APC are postulated to be responsible for the therapeutic benefit of APC in the treatment of severe sepsis.
Figures
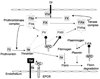
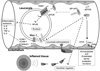

Similar articles
-
Protective mechanisms of activated protein C in severe inflammatory disorders.Br J Pharmacol. 2009 Oct;158(4):1034-47. doi: 10.1111/j.1476-5381.2009.00251.x. Epub 2009 May 14. Br J Pharmacol. 2009. PMID: 19466992 Free PMC article. Review.
-
Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions.J Biol Chem. 2007 Nov 9;282(45):33022-33. doi: 10.1074/jbc.M705824200. Epub 2007 Sep 14. J Biol Chem. 2007. PMID: 17872949
-
Activated protein C and sepsis.Front Biosci. 2006 Jan 1;11:676-98. doi: 10.2741/1827. Front Biosci. 2006. PMID: 16146761 Review.
-
Identification of a specific exosite on activated protein C for interaction with protease-activated receptor 1.J Biol Chem. 2007 Aug 31;282(35):25493-500. doi: 10.1074/jbc.M702131200. Epub 2007 Jun 19. J Biol Chem. 2007. PMID: 17580306
-
Protein C in critical illness.Am J Health Syst Pharm. 2009 Jun 15;66(12):1089-96. doi: 10.2146/ajhp080276. Am J Health Syst Pharm. 2009. PMID: 19498123 Review.
Cited by
-
Histone cleavage as a mechanism for epigenetic regulation: current insights and perspectives.Curr Mol Med. 2014;14(9):1164-72. doi: 10.2174/1566524014666141015155630. Curr Mol Med. 2014. PMID: 25323999 Free PMC article. Review.
-
Mimicking immune complexes for efficient antibody responses.Front Immunol. 2025 Apr 28;16:1570487. doi: 10.3389/fimmu.2025.1570487. eCollection 2025. Front Immunol. 2025. PMID: 40356891 Free PMC article.
-
Plasma carboxypeptidase B downregulates inflammatory responses in autoimmune arthritis.J Clin Invest. 2011 Sep;121(9):3517-27. doi: 10.1172/JCI46387. Epub 2011 Aug 1. J Clin Invest. 2011. PMID: 21804193 Free PMC article.
-
Validation for the function of protein C in mouse models.PeerJ. 2024 Apr 24;12:e17261. doi: 10.7717/peerj.17261. eCollection 2024. PeerJ. 2024. PMID: 38680896 Free PMC article.
-
Activated Protein C Attenuates Severe Inflammation by Targeting VLA-3high Neutrophil Subpopulation in Mice.J Immunol. 2017 Oct 15;199(8):2930-2936. doi: 10.4049/jimmunol.1700541. Epub 2017 Sep 6. J Immunol. 2017. PMID: 28877991 Free PMC article.
References
-
- Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, Beale R, Svoboda P, Laterre PF, Simon S, Light B, Spapen H, Stone J, Seibert A, Peckelsen C, De Deyne C, Postier R, Pettila V, Artigas A, Percell SR, Shu V, Zwingelstein C, Tobias J, Poole L, Stolzenbach JC, Creasey AA. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. Jama. 2003;290:238–247. - PubMed
-
- Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, Francois B, Guy JS, Bruckmann M, Rea-Neto A, Rossaint R, Perrotin D, Sablotzki A, Arkins N, Utterback BG, Macias WL. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005;353:1332–1341. - PubMed
-
- Aoki T, Suzuki Y, Nishio K, Suzuki K, Miyata A, Iigou Y, Serizawa H, Tsumura H, Ishimura Y, Suematsu M, Yamaguchi K. Role of CD18-ICAM-1 in the entrapment of stimulated leukocytes in alveolar capillaries of perfused rat lungs. Am J Physiol. 1997;273:H2361–H2371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

