Interaction of influenza virus NS1 protein with growth arrest-specific protein 8
- PMID: 19995461
- PMCID: PMC2797798
- DOI: 10.1186/1743-422X-6-218
Interaction of influenza virus NS1 protein with growth arrest-specific protein 8
Abstract
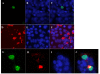
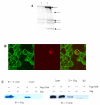
NS1 protein is the only non-structural protein encoded by the influenza A virus, and it contributes significantly to disease pathogenesis by modulating many virus and host cell processes. A two-hybrid screen for proteins that interact with NS1 from influenza A yielded growth arrest-specific protein 8. Gas8 associated with NS1 in vitro and in vivo. Deletion analysis revealed that the N-terminal 260 amino acids of Gas8 were able to interact with NS1, and neither the RNA-binding domain nor the effector domain of NS1 was sufficient for the NS1 interaction. We also found that actin, myosin, and drebrin interact with Gas8. NS1 and beta-actin proteins could be co-immunoprecipitated from extracts of transfected cells. Furthermore, actin and Gas8 co-localized at the plasma membrane. These results are discussed in relation to the possible functions of Gas8 protein and their relevance in influenza virus release.
Figures


Similar articles
-
Interaction of influenza virus NS1 protein and the human homologue of Staufen in vivo and in vitro.Nucleic Acids Res. 1999 Jun 1;27(11):2241-7. doi: 10.1093/nar/27.11.2241. Nucleic Acids Res. 1999. PMID: 10325410 Free PMC article.
-
Heterologous interactions between NS1 proteins from different influenza A virus subtypes/strains.Sci China Life Sci. 2012 Jun;55(6):507-15. doi: 10.1007/s11427-012-4335-6. Epub 2012 Jun 29. Sci China Life Sci. 2012. PMID: 22744180
-
A Naturally Occurring Deletion in the Effector Domain of H5N1 Swine Influenza Virus Nonstructural Protein 1 Regulates Viral Fitness and Host Innate Immunity.J Virol. 2018 May 14;92(11):e00149-18. doi: 10.1128/JVI.00149-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29563291 Free PMC article.
-
The non-structural (NS1) protein of influenza A virus associates with p53 and inhibits p53-mediated transcriptional activity and apoptosis.Biochem Biophys Res Commun. 2010 Apr 23;395(1):141-5. doi: 10.1016/j.bbrc.2010.03.160. Epub 2010 Mar 31. Biochem Biophys Res Commun. 2010. PMID: 20361939
-
The influenza virus NS1 protein: inhibitor of innate and adaptive immunity.Infect Disord Drug Targets. 2007 Dec;7(4):336-43. doi: 10.2174/187152607783018754. Infect Disord Drug Targets. 2007. PMID: 18220965 Review.
Cited by
-
Interaction of avian influenza virus NS1 protein and nucleolar and coiled-body phosphoprotein 1.Virus Genes. 2013 Apr;46(2):287-92. doi: 10.1007/s11262-012-0849-z. Epub 2012 Nov 28. Virus Genes. 2013. PMID: 23188192 Free PMC article.
-
The influenza virus NS1 protein as a therapeutic target.Antiviral Res. 2013 Sep;99(3):409-16. doi: 10.1016/j.antiviral.2013.06.005. Epub 2013 Jun 21. Antiviral Res. 2013. PMID: 23796981 Free PMC article. Review.
-
Detecting protein-protein interactions in vesicular stomatitis virus using a cytoplasmic yeast two hybrid system.J Virol Methods. 2011 May;173(2):203-12. doi: 10.1016/j.jviromet.2011.02.006. Epub 2011 Feb 12. J Virol Methods. 2011. PMID: 21320532 Free PMC article.
-
Roles of NOLC1 in cancers and viral infection.J Cancer Res Clin Oncol. 2023 Sep;149(12):10593-10608. doi: 10.1007/s00432-023-04934-5. Epub 2023 Jun 9. J Cancer Res Clin Oncol. 2023. PMID: 37296317 Free PMC article. Review.
-
The potential role of microfilaments in host cells for infection with infectious spleen and kidney necrosis virus infection.Virol J. 2013 Mar 7;10:77. doi: 10.1186/1743-422X-10-77. Virol J. 2013. PMID: 23497248 Free PMC article.
References
-
- Marion RM, Zurcher T, Luna S, Ortin J. Influenza virus NS1 protein interacts with viral transcription-replication complexes in vivo. J Gen Virol. 1997;78:2447–2451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

