Fibroblasts and myofibroblasts in wound healing: force generation and measurement
- PMID: 19995679
- PMCID: PMC2891362
- DOI: 10.1016/j.jtv.2009.11.004
Fibroblasts and myofibroblasts in wound healing: force generation and measurement
Abstract
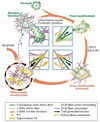
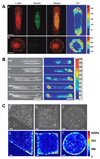
Fibroblasts are one of the most abundant cell types in connective tissues. These cells are responsible for tissue homeostasis under normal physiological conditions. When tissues are injured, fibroblasts become activated and differentiate into myofibroblasts, which generate large contractions and actively produce extracellular matrix (ECM) proteins to facilitate wound closure. Both fibroblasts and myofibroblasts play a critical role in wound healing by generating traction and contractile forces, respectively, to enhance wound contraction. This review focuses on the mechanisms of force generation in fibroblasts and myofibroblasts and techniques for measuring such cellular forces. Such a topic was chosen specifically because of the dual effects that fibroblasts/myofibroblasts have in wound healing process- a suitable amount of force generation and matrix deposition is beneficial for wound healing; excessive force and matrix production, however, result in tissue scarring and even malfunction of repaired tissues. Therefore, understanding how forces are generated in these cells and knowing exactly how much force they produce may guide the development of optimal protocols for more effective treatment of tissue wounds in clinical settings.
Copyright © 2009 Tissue Viability Society. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Nat Rev Mol Cell Biol. 2002;3:349–363. - PubMed
-
- Kozma EM, Olczyk K, Bobinski R, Kasperczyk M, Szpyra K. Chir Narzadow Ruchu Ortop Pol. 2002;67:73–79. - PubMed
-
- Hinz B. J Invest Dermatol. 2007;127:526–537. - PubMed
-
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Am J Physiol. 1999;277:C1–C9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

