Molecular characterization of the Calvin cycle enzyme phosphoribulokinase in the stramenopile alga Vaucheria litorea and the plastid hosting mollusc Elysia chlorotica
- PMID: 19995736
- PMCID: PMC2782795
- DOI: 10.1093/mp/ssp085
Molecular characterization of the Calvin cycle enzyme phosphoribulokinase in the stramenopile alga Vaucheria litorea and the plastid hosting mollusc Elysia chlorotica
Abstract
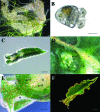
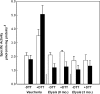
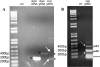
Phosphoribulokinase (PRK), a nuclear-encoded plastid-localized enzyme unique to the photosynthetic carbon reduction (Calvin) cycle, was cloned and characterized from the stramenopile alga Vaucheria litorea. This alga is the source of plastids for the mollusc (sea slug) Elysia chlorotica which enable the animal to survive for months solely by photoautotrophic CO2 fixation. The 1633-bp V. litorea prk gene was cloned and the coding region, found to be interrupted by four introns, encodes a 405-amino acid protein. This protein contains the typical bipartite target sequence expected of nuclear-encoded proteins that are directed to complex (i.e. four membrane-bound) algal plastids. De novo synthesis of PRK and enzyme activity were detected in E. chlorotica in spite of having been starved of V. litorea for several months. Unlike the algal enzyme, PRK in the sea slug did not exhibit redox regulation. Two copies of partial PRK-encoding genes were isolated from both sea slug and aposymbiotic sea slug egg DNA using PCR. Each copy contains the nucleotide region spanning exon 1 and part of exon 2 of V. litorea prk, including the bipartite targeting peptide. However, the larger prk fragment also includes intron 1. The exon and intron sequences of prk in E. chlorotica and V. litorea are nearly identical. These data suggest that PRK is differentially regulated in V. litorea and E. chlorotica and at least a portion of the V. litorea nuclear PRK gene is present in sea slugs that have been starved for several months.
Keywords: Alga; Calvin cycle; Elysia chlorotica; Vaucheria litorea; kleptoplast; mollusc; phosphoribulokinase; photosynthesis; plastid; redox regulation; stramenopile; symbiosis.
Figures


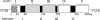

References
-
- Armbrust EV, et al. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science. 2004;306:79–86. - PubMed
-
- Bhattacharya D, Yoon HS, Hackett JD. Photosynthetic eukaryotes unite: endosymbiosis connects the dots. Bioessays. 2004;26:50–60. - PubMed
-
- Bock R, Timmis JN. Reconstructing evolution: gene transfer from plastids to the nucleus. Bioessays. 2008;30:556–566. - PubMed
-
- Boggetto N, Gontero B, Maberly SC. Regulation of phosphoribulokinase and glyceraldehyde 3-phosphate dehydrogenase in a freshwater diatom, Asterionella formosa. J. Phycol. 2007;43:1227–1235. - PubMed
-
- Chen XF, et al. Molecular cloning and expression analysis of rice phosphoribulokinase gene that is regulated by environmental stresses. Mol. Biol. Rep. 2004;31:249–255. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

