Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis
- PMID: 19995812
- PMCID: PMC2790574
- DOI: 10.1136/bmj.b5106
Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis
Abstract
Objectives: To update a 2005 Cochrane review that assessed the effects of neuraminidase inhibitors in preventing or ameliorating the symptoms of influenza, the transmission of influenza, and complications from influenza in healthy adults, and to estimate the frequency of adverse effects. Search strategy An updated search of the Cochrane central register of controlled trials (Cochrane Library 2009, issue 2), which contains the Acute Respiratory Infections Group's specialised register, Medline (1950-Aug 2009), Embase (1980-Aug 2009), and post-marketing pharmacovigilance data and comparative safety cohorts. Selection criteria Randomised placebo controlled studies of neuraminidase inhibitors in otherwise healthy adults exposed to naturally occurring influenza.
Main outcome measures: Duration and incidence of symptoms; incidence of lower respiratory tract infections, or their proxies; and adverse events.
Data extraction: Two reviewers applied inclusion criteria, assessed trial quality, and extracted data. Data analysis Comparisons were structured into prophylaxis, treatment, and adverse events, with further subdivision by outcome and dose.
Results: 20 trials were included: four on prophylaxis, 12 on treatment, and four on postexposure prophylaxis. For prophylaxis, neuraminidase inhibitors had no effect against influenza-like illness or asymptomatic influenza. The efficacy of oral oseltamivir against symptomatic laboratory confirmed influenza was 61% (risk ratio 0.39, 95% confidence interval 0.18 to 0.85) at 75 mg daily and 73% (0.27, 0.11 to 0.67) at 150 mg daily. Inhaled zanamivir 10 mg daily was 62% efficacious (0.38, 0.17 to 0.85). Oseltamivir for postexposure prophylaxis had an efficacy of 58% (95% confidence interval 15% to 79%) and 84% (49% to 95%) in two trials of households. Zanamivir performed similarly. The hazard ratios for time to alleviation of influenza-like illness symptoms were in favour of treatment: 1.20 (95% confidence interval 1.06 to 1.35) for oseltamivir and 1.24 (1.13 to 1.36) for zanamivir. Eight unpublished studies on complications were ineligible and therefore excluded. The remaining evidence suggests oseltamivir did not reduce influenza related lower respiratory tract complications (risk ratio 0.55, 95% confidence interval 0.22 to 1.35). From trial evidence, oseltamivir induced nausea (odds ratio 1.79, 95% confidence interval 1.10 to 2.93). Evidence of rarer adverse events from pharmacovigilance was of poor quality or possibly under-reported.
Conclusion: Neuraminidase inhibitors have modest effectiveness against the symptoms of influenza in otherwise healthy adults. The drugs are effective postexposure against laboratory confirmed influenza, but this is a small component of influenza-like illness, so for this outcome neuraminidase inhibitors are not effective. Neuraminidase inhibitors might be regarded as optional for reducing the symptoms of seasonal influenza. Paucity of good data has undermined previous findings for oseltamivir's prevention of complications from influenza. Independent randomised trials to resolve these uncertainties are needed.
Conflict of interest statement
Competing interests: All authors have completed the unified competing interest form at
Figures
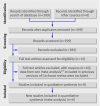
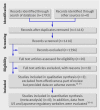





Comment in
-
Why don't we have all the evidence on oseltamivir?BMJ. 2009 Dec 8;339:b5351. doi: 10.1136/bmj.b5351. BMJ. 2009. PMID: 19995815 No abstract available.
-
The truth about Tamiflu? Neuraminidase inhibitors in pandemic A/H1N1 flu.BMJ. 2010 Jan 13;340:c130. doi: 10.1136/bmj.c130. BMJ. 2010. PMID: 20071425 No abstract available.
-
The truth about Tamiflu? Zanamivir should be inhaled, not nebulised.BMJ. 2010 Jan 13;340:c131. doi: 10.1136/bmj.c131. BMJ. 2010. PMID: 20071426 Free PMC article. No abstract available.
-
ACP Journal Club. Review: Neuraminidase inhibitors relieve influenza symptoms and reduce laboratory-confirmed influenza in healthy adults.Ann Intern Med. 2010 Feb 16;152(4):JC-211. doi: 10.7326/0003-4819-152-4-201002160-02011. Ann Intern Med. 2010. PMID: 20157123 No abstract available.
References
-
- Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis 2009;48(suppl 1):S3-13. - PubMed
-
- Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med 2005;353:1363-73. - PubMed
-
- World Health Organization. Global agenda on influenza surveillance and control. 2002. www.who.int/csr/disease/influenza/csrinfluenzaglobalagenda/en/print.html.
-
- Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al. Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation. Health Technol Assess 2002;6:1-87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
