Atomic structure and dynamics of pentameric ligand-gated ion channels: new insight from bacterial homologues
- PMID: 19995852
- PMCID: PMC2828131
- DOI: 10.1113/jphysiol.2009.183160
Atomic structure and dynamics of pentameric ligand-gated ion channels: new insight from bacterial homologues
Abstract
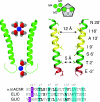
Pentameric ligand-gated ion channels (pLGICs) are widely expressed in the animal kingdom and are key players of neurotransmission by acetylcholine (ACh), gamma-amminobutyric acid (GABA), glycine and serotonin. It is now established that this family has a prokaryotic origin, since more than 20 homologues have been discovered in bacteria. In particular, the GLIC homologue displays a ligand-gated ion channel function and is activated by protons. The prokaryotic origin of these membrane proteins facilitated the X-ray structural resolution of the first members of this family. ELIC was solved at 3.3 A in a closed-pore conformation, and GLIC at up to 2.9 A in an apparently open-pore conformation. These data reveal many structural features, notably the architecture of the pore, including its gate and its selectivity filter, and the interactions between the protein and lipids. In addition, comparison of the structures of GLIC and ELIC hints at a mechanism of channel opening, which consists of both a quaternary twist and a tertiary deformation. This mechanism couples opening-closing motions of the channel with a global reorganization of the protein, including the subunit interface that holds the neurotransmitter binding sites in eukaryotic pLGICs.
Figures

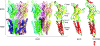
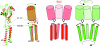

References
-
- Beckstein O, Sansom MSP. A hydrophobic gate in an ion channel: the closed state of the nicotinic acetylcholine receptor. Phys Biol. 2006;3:147–159. - PubMed
-
- Bouzat C, Gumilar F, Spitzmaul G, Wang H, Rayes D, Hansen SB, Taylor P, Sine SM. Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature. 2004;430:896–900. - PubMed
-
- Bocquet N, Prado de Carvalho L, Cartaud J, Neyton J, Poupon CL, Taly A, Grutter T, Changeux J, Corringer PJ. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007;445:116–119. - PubMed
-
- Bocquet N, Nury H, Baaden M, Poupon CL, Changeux J, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

