STAT3 signaling in CD4+ T cells is critical for the pathogenesis of chronic sclerodermatous graft-versus-host disease in a murine model
- PMID: 19995899
- PMCID: PMC2941975
- DOI: 10.4049/jimmunol.0903006
STAT3 signaling in CD4+ T cells is critical for the pathogenesis of chronic sclerodermatous graft-versus-host disease in a murine model
Abstract
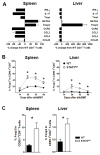
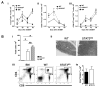
Donor CD4+ T cells are thought to be essential for inducing delayed host tissue injury in chronic graft-versus-host disease (GVHD). However, the relative contributions of distinct effector CD4+ T cell subpopulations and the molecular pathways influencing their generation are not known. We investigated the role of the STAT3 pathway in a murine model of chronic sclerodermatous GVHD. This pathway integrates multiple signaling events during the differentiation of naive CD4+ T cells and impacts their homeostasis. We report that chimeras receiving an allograft containing STAT3-ablated donor CD4+ T cells do not develop classic clinical and pathological manifestations of alloimmune tissue injury. Analysis of chimeras showed that abrogation of STAT3 signaling reduced the in vivo expansion of donor-derived CD4+ T cells and their accumulation in GVHD target tissues without abolishing antihost alloreactivity. STAT3 ablation did not significantly affect Th1 differentiation while enhancing CD4+CD25+Foxp3+ T cell reconstitution through thymus-dependent and -independent pathways. Transient depletion of CD25+ T cells in chimeras receiving STAT3-deficient T cells resulted in delayed development of alloimmune gut and liver injury. This delayed de novo GVHD was associated with the emergence of donor hematopoietic stem cell-derived Th1 and Th17 cells. These results suggest that STAT3 signaling in graft CD4+ T cells links the alloimmune tissue injury of donor graft T cells and the emergence of donor hematopoietic stem cell-derived pathogenic effector cells and that both populations contribute, albeit in different ways, to the genesis of chronic GVHD after allogenic bone marrow transplantation in a murine model.
Figures

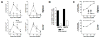

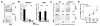

Similar articles
-
PIAS3 suppresses acute graft-versus-host disease by modulating effector T and B cell subsets through inhibition of STAT3 activation.Immunol Lett. 2014 Jul;160(1):79-88. doi: 10.1016/j.imlet.2014.03.014. Epub 2014 Apr 6. Immunol Lett. 2014. PMID: 24718277
-
Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: implications in graft-versus-host disease and antitumor immunity.J Immunol. 2007 Dec 1;179(11):7593-604. doi: 10.4049/jimmunol.179.11.7593. J Immunol. 2007. PMID: 18025205
-
STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease.Immunity. 2012 Aug 24;37(2):209-22. doi: 10.1016/j.immuni.2012.05.027. Immunity. 2012. PMID: 22921119 Free PMC article.
-
Modulation of graft-versus-host disease: role of regulatory T lymphocytes.Biol Blood Marrow Transplant. 2006 Jan;12(1 Suppl 2):13-21. doi: 10.1016/j.bbmt.2005.11.002. Biol Blood Marrow Transplant. 2006. PMID: 16399597 Review.
-
Biology of chronic graft-versus-host disease: implications for a future therapeutic approach.Keio J Med. 2008 Dec;57(4):177-83. doi: 10.2302/kjm.57.177. Keio J Med. 2008. PMID: 19110529 Free PMC article. Review.
Cited by
-
BET-bromodomain and EZH2 inhibitor-treated chronic GVHD mice have blunted germinal centers with distinct transcriptomes.Blood. 2022 May 12;139(19):2983-2997. doi: 10.1182/blood.2021014557. Blood. 2022. PMID: 35226736 Free PMC article.
-
β2-Adrenergic receptor activation on donor cells ameliorates acute GvHD.JCI Insight. 2020 Jun 18;5(12):e137788. doi: 10.1172/jci.insight.137788. JCI Insight. 2020. PMID: 32437333 Free PMC article.
-
Dissecting the regulatory network of transcription factors in T cell phenotype/functioning during GVHD and GVT.Front Immunol. 2023 Jun 27;14:1194984. doi: 10.3389/fimmu.2023.1194984. eCollection 2023. Front Immunol. 2023. PMID: 37441063 Free PMC article. Review.
-
Advances in graft-versus-host disease biology and therapy.Nat Rev Immunol. 2012 May 11;12(6):443-58. doi: 10.1038/nri3212. Nat Rev Immunol. 2012. PMID: 22576252 Free PMC article. Review.
-
Tumor microenvironment macrophage inhibitory factor directs the accumulation of interleukin-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients.J Biol Chem. 2012 Oct 12;287(42):35484-35495. doi: 10.1074/jbc.M112.367532. Epub 2012 Aug 14. J Biol Chem. 2012. PMID: 22893706 Free PMC article.
References
-
- Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. - PubMed
-
- Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. - PubMed
-
- Parkman R. Is chronic graft versus host disease an autoimmune disease? Curr Opin Immunol. 1993;5:800–803. - PubMed
-
- Zhang Y, Hexner E, Frank D, Emerson SG. CD4+ T Cells Generated De Novo from Donor Hemopoietic Stem Cells Mediate the Evolution from Acute to Chronic Graft-versus-Host Disease. J Immunol. 2007;179:3305–3314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

