Telomere protection by TPP1 is mediated by POT1a and POT1b
- PMID: 19995905
- PMCID: PMC2815557
- DOI: 10.1128/MCB.01498-09
Telomere protection by TPP1 is mediated by POT1a and POT1b
Abstract
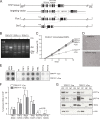
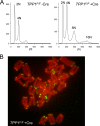
Mammalian telomeres are protected by the shelterin complex, which contains single-stranded telomeric DNA binding proteins (POT1a and POT1b in rodents, POT1 in other mammals). Mouse POT1a prevents the activation of the ATR kinase and contributes to the repression of the nonhomologous end-joining pathway (NHEJ) at newly replicated telomeres. POT1b represses unscheduled resection of the 5'-ended telomeric DNA strand, resulting in long 3' overhangs in POT1b KO cells. Both POT1 proteins bind TPP1, forming heterodimers that bind to other proteins in shelterin. Short hairpin RNA (shRNA)-mediated depletion had previously demonstrated that TPP1 contributes to the normal function of POT1a and POT1b. However, these experiments did not establish whether TPP1 has additional functions in shelterin. Here we report on the phenotypes of the conditional deletion of TPP1 from mouse embryo fibroblasts. TPP1 deletion resulted in the release of POT1a and POT1b from chromatin and loss of these proteins from telomeres, indicating that TPP1 is required for the telomere association of POT1a and POT1b but not for their stability. The telomere dysfunction phenotypes associated with deletion of TPP1 were identical to those of POT1a/POT1b DKO cells. No additional telomere dysfunction phenotypes were observed, establishing that the main role of TPP1 is to allow POT1a and POT1b to protect chromosome ends.
Figures



Similar articles
-
Telomere protection by TPP1/POT1 requires tethering to TIN2.Mol Cell. 2011 Nov 18;44(4):647-59. doi: 10.1016/j.molcel.2011.08.043. Mol Cell. 2011. PMID: 22099311 Free PMC article.
-
Protection of telomeres 1 proteins POT1a and POT1b can repress ATR signaling by RPA exclusion, but binding to CST limits ATR repression by POT1b.J Biol Chem. 2018 Sep 14;293(37):14384-14392. doi: 10.1074/jbc.RA118.004598. Epub 2018 Aug 6. J Biol Chem. 2018. PMID: 30082315 Free PMC article.
-
Binding of TPP1 protein to TIN2 protein is required for POT1a,b protein-mediated telomere protection.J Biol Chem. 2014 Aug 29;289(35):24180-7. doi: 10.1074/jbc.M114.592592. Epub 2014 Jul 23. J Biol Chem. 2014. PMID: 25056954 Free PMC article.
-
POT1-TPP1 telomere length regulation and disease.Comput Struct Biotechnol J. 2020 Jul 3;18:1939-1946. doi: 10.1016/j.csbj.2020.06.040. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32774788 Free PMC article. Review.
-
Shelterin: the protein complex that shapes and safeguards human telomeres.Genes Dev. 2005 Sep 15;19(18):2100-10. doi: 10.1101/gad.1346005. Genes Dev. 2005. PMID: 16166375 Review.
Cited by
-
Shelterin Complex at Telomeres: Implications in Ageing.Clin Interv Aging. 2020 Jun 3;15:827-839. doi: 10.2147/CIA.S256425. eCollection 2020. Clin Interv Aging. 2020. PMID: 32581523 Free PMC article. Review.
-
The Pot1a-associated proteins Tpt1 and Pat1 coordinate telomere protection and length regulation in Tetrahymena.Mol Biol Cell. 2011 Nov;22(21):4161-70. doi: 10.1091/mbc.E11-06-0551. Epub 2011 Sep 7. Mol Biol Cell. 2011. PMID: 21900503 Free PMC article.
-
Crosstalk between telomere maintenance and radiation effects: A key player in the process of radiation-induced carcinogenesis.Mutat Res Rev Mutat Res. 2014 Jan 31:S1383-5742(14)00002-7 10.1016/j.mrrev.2014.01.001. doi: 10.1016/j.mrrev.2014.01.001. Online ahead of print. Mutat Res Rev Mutat Res. 2014. PMID: 24486376 Free PMC article. Review.
-
To Join or Not to Join: Decision Points Along the Pathway to Double-Strand Break Repair vs. Chromosome End Protection.Front Cell Dev Biol. 2021 Jul 12;9:708763. doi: 10.3389/fcell.2021.708763. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34322492 Free PMC article. Review.
-
TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo.Mol Cell Biol. 2010 Jun;30(12):2971-82. doi: 10.1128/MCB.00240-10. Epub 2010 Apr 19. Mol Cell Biol. 2010. PMID: 20404094 Free PMC article.
References
-
- Baumann, P., and T. R. Cech. 2001. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292:1171-1175. - PubMed
-
- Celli, G., and T. de Lange. 2005. DNA processing not required for ATM-mediated telomere damage response after TRF2 deletion. Nat. Cell Biol. 7:712-718. - PubMed
-
- de Lange, T. 2005. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19:2100-2110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
