Modulation of chromatin boundary activities by nucleosome-remodeling activities in Drosophila melanogaster
- PMID: 19995906
- PMCID: PMC2815568
- DOI: 10.1128/MCB.00183-09
Modulation of chromatin boundary activities by nucleosome-remodeling activities in Drosophila melanogaster
Abstract
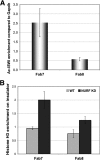
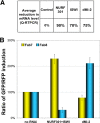
Chromatin boundaries facilitate independent gene regulation by insulating genes from the effects of enhancers or organized chromatin. However, the mechanisms of boundary action are not well understood. To investigate whether boundary function depends on a higher order of chromatin organization, we examined the function of several Drosophila melanogaster insulators in cells with reduced chromatin-remodeling activities. We found that knockdown of NURF301 and ISWI, key components of the nucleosome-remodeling factor (NURF), synergistically disrupted the enhancer-blocking function of Fab7 and SF1 and augmented the function of Fab8. Mutations in Nurf301/Ebx and Iswi also affected the function of these boundaries in vivo. We further show that ISWI was localized on the endogenous Fab7 and Fab8 insulators and that NURF knockdown resulted in a marked increase in the nucleosome occupancy at these insulator sites. In contrast to the effect of NURF knockdown, reduction in dMi-2, the ATPase component of the Drosophila nucleosome-remodeling and deacetylation (NuRD) complex, augmented Fab7 and suppressed Fab8. Our results provide the first evidence that higher-order chromatin organization influences the enhancer-blocking activity of chromatin boundaries. In particular, the NURF and NuRD nucleosome-remodeling complexes may regulate Hox expression by modulating the function of boundaries in these complexes. The unique responses by different classes of boundaries to changes in the chromatin environment may be indicative of their distinct mechanisms of action, which may influence their placement in the genome and selection during evolution.
Figures



Similar articles
-
The nucleosome remodeling factor.FEBS Lett. 2011 Oct 20;585(20):3197-207. doi: 10.1016/j.febslet.2011.09.003. Epub 2011 Sep 9. FEBS Lett. 2011. PMID: 21920360 Free PMC article. Review.
-
Genome-Wide Mapping Targets of the Metazoan Chromatin Remodeling Factor NURF Reveals Nucleosome Remodeling at Enhancers, Core Promoters and Gene Insulators.PLoS Genet. 2016 Apr 5;12(4):e1005969. doi: 10.1371/journal.pgen.1005969. eCollection 2016 Apr. PLoS Genet. 2016. PMID: 27046080 Free PMC article.
-
Alternative splicing of NURF301 generates distinct NURF chromatin remodeling complexes with altered modified histone binding specificities.PLoS Genet. 2009 Jul;5(7):e1000574. doi: 10.1371/journal.pgen.1000574. Epub 2009 Jul 24. PLoS Genet. 2009. PMID: 19629165 Free PMC article.
-
Biological functions of the ISWI chromatin remodeling complex NURF.Genes Dev. 2002 Dec 15;16(24):3186-98. doi: 10.1101/gad.1032202. Genes Dev. 2002. PMID: 12502740 Free PMC article.
-
GAGA factor: a multifunctional pioneering chromatin protein.Cell Mol Life Sci. 2021 May;78(9):4125-4141. doi: 10.1007/s00018-021-03776-z. Epub 2021 Feb 2. Cell Mol Life Sci. 2021. PMID: 33528710 Free PMC article. Review.
Cited by
-
Dynamic nucleosome organization at hox promoters during zebrafish embryogenesis.PLoS One. 2013 May 9;8(5):e63175. doi: 10.1371/journal.pone.0063175. Print 2013. PLoS One. 2013. PMID: 23671670 Free PMC article.
-
The nucleosome remodeling factor.FEBS Lett. 2011 Oct 20;585(20):3197-207. doi: 10.1016/j.febslet.2011.09.003. Epub 2011 Sep 9. FEBS Lett. 2011. PMID: 21920360 Free PMC article. Review.
-
Insulators and promoters: closer than we think.Nat Rev Genet. 2010 Jun;11(6):439-46. doi: 10.1038/nrg2765. Epub 2010 May 5. Nat Rev Genet. 2010. PMID: 20442713 Free PMC article. Review.
-
A functional insulator screen identifies NURF and dREAM components to be required for enhancer-blocking.PLoS One. 2014 Sep 23;9(9):e107765. doi: 10.1371/journal.pone.0107765. eCollection 2014. PLoS One. 2014. PMID: 25247414 Free PMC article.
-
Surviving an identity crisis: a revised view of chromatin insulators in the genomics era.Biochim Biophys Acta. 2014 Mar;1839(3):203-14. doi: 10.1016/j.bbagrm.2013.10.007. Epub 2013 Nov 1. Biochim Biophys Acta. 2014. PMID: 24189492 Free PMC article. Review.
References
-
- Adkins, N. L., T. A. Hagerman, and P. Georgel. 2006. GAGA protein: a multi-faceted transcription factor. Biochem. Cell. Biol. 84:559-567. - PubMed
-
- Avramova, Z., and A. Tikhonov. 1999. Are scs and scs′ ‘neutral’ chromatin domain boundaries of the locus? Trends Genet. 15:138-139. - PubMed
-
- Babu, P., K. S. Selvakumar, and S. Bhosekar. 1987. Studies on transvection at the bithorax complex in Drosophila melanogaster. Mol. Gen. Genet. 210:557-563. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
