mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling
- PMID: 19995915
- PMCID: PMC2815569
- DOI: 10.1128/MCB.00601-09
mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling
Abstract
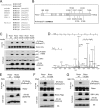
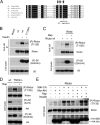
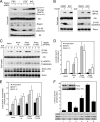
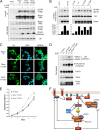
The mammalian target of rapamycin (mTOR) is a conserved Ser/Thr kinase that forms two functionally distinct complexes important for nutrient and growth factor signaling. While mTOR complex 1 (mTORC1) regulates mRNA translation and ribosome biogenesis, mTORC2 plays an important role in the phosphorylation and subsequent activation of Akt. Interestingly, mTORC1 negatively regulates Akt activation, but whether mTORC1 signaling directly targets mTORC2 remains unknown. Here we show that growth factors promote the phosphorylation of Rictor (rapamycin-insensitive companion of mTOR), an essential subunit of mTORC2. We found that Rictor phosphorylation requires mTORC1 activity and, more specifically, the p70 ribosomal S6 kinase 1 (S6K1). We identified several phosphorylation sites in Rictor and found that Thr1135 is directly phosphorylated by S6K1 in vitro and in vivo, in a rapamycin-sensitive manner. Phosphorylation of Rictor on Thr1135 did not affect mTORC2 assembly, kinase activity, or cellular localization. However, cells expressing a Rictor T1135A mutant were found to have increased mTORC2-dependent phosphorylation of Akt. In addition, phosphorylation of the Akt substrates FoxO1/3a and glycogen synthase kinase 3 alpha/beta (GSK3 alpha/beta) was found to be increased in these cells, indicating that S6K1-mediated phosphorylation of Rictor inhibits mTORC2 and Akt signaling. Together, our results uncover a new regulatory link between the two mTOR complexes, whereby Rictor integrates mTORC1-dependent signaling.
Figures


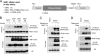
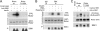
Similar articles
-
Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1.Mol Cell Biol. 2009 Nov;29(21):5657-70. doi: 10.1128/MCB.00735-09. Epub 2009 Aug 31. Mol Cell Biol. 2009. PMID: 19720745 Free PMC article.
-
Receptor-recognized α₂-macroglobulin binds to cell surface-associated GRP78 and activates mTORC1 and mTORC2 signaling in prostate cancer cells.PLoS One. 2012;7(12):e51735. doi: 10.1371/journal.pone.0051735. Epub 2012 Dec 14. PLoS One. 2012. Retraction in: PLoS One. 2025 Jun 5;20(6):e0325675. doi: 10.1371/journal.pone.0325675. PMID: 23272152 Free PMC article. Retracted.
-
PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling.J Biol Chem. 2007 Aug 31;282(35):25604-12. doi: 10.1074/jbc.M704343200. Epub 2007 Jun 28. J Biol Chem. 2007. PMID: 17599906
-
The complexes of mammalian target of rapamycin.Curr Protein Pept Sci. 2010 Sep;11(6):409-24. doi: 10.2174/138920310791824093. Curr Protein Pept Sci. 2010. PMID: 20491627 Free PMC article. Review.
-
A complex interplay between Akt, TSC2 and the two mTOR complexes.Biochem Soc Trans. 2009 Feb;37(Pt 1):217-22. doi: 10.1042/BST0370217. Biochem Soc Trans. 2009. PMID: 19143635 Free PMC article. Review.
Cited by
-
The multifaceted role of mTORC1 in the control of lipid metabolism.EMBO Rep. 2013 Mar 1;14(3):242-51. doi: 10.1038/embor.2013.5. Epub 2012 Feb 12. EMBO Rep. 2013. PMID: 23399656 Free PMC article. Review.
-
Interaction of polyamines and mTOR signaling in the synthesis of antizyme (AZ).Cell Signal. 2015 Sep;27(9):1850-9. doi: 10.1016/j.cellsig.2015.06.002. Epub 2015 Jun 17. Cell Signal. 2015. PMID: 26093026 Free PMC article.
-
S6 kinase 1 at the central node of cell size and ageing.Front Cell Dev Biol. 2022 Aug 12;10:949196. doi: 10.3389/fcell.2022.949196. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36036012 Free PMC article. Review.
-
Radiosensitization of Glioblastoma Cell Lines by the Dual PI3K and mTOR Inhibitor NVP-BEZ235 Depends on Drug-Irradiation Schedule.Transl Oncol. 2013 Apr;6(2):169-79. doi: 10.1593/tlo.12364. Epub 2013 Apr 1. Transl Oncol. 2013. PMID: 23544169 Free PMC article.
-
TSC1 controls IL-1β expression in macrophages via mTORC1-dependent C/EBPβ pathway.Cell Mol Immunol. 2016 Sep;13(5):640-50. doi: 10.1038/cmi.2015.43. Epub 2015 May 25. Cell Mol Immunol. 2016. PMID: 27593484 Free PMC article.
References
-
- Alessi, D. R., F. B. Caudwell, M. Andjelkovic, B. A. Hemmings, and P. Cohen. 1996. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399:333-338. - PubMed
-
- Alessi, D. R., L. R. Pearce, and J. M. Garcia-Martinez. 2009. New insights into mTOR signaling: mTORC2 and beyond. Sci. Signal 2:pe27. - PubMed
-
- Baldo, P., S. Cecco, E. Giacomin, R. Lazzarini, B. Ros, and S. Marastoni. 2008. mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr. Cancer Drug Targets 8:647-665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
