SRC-mediated phosphorylation of dynamin and cortactin regulates the "constitutive" endocytosis of transferrin
- PMID: 19995918
- PMCID: PMC2812239
- DOI: 10.1128/MCB.00330-09
SRC-mediated phosphorylation of dynamin and cortactin regulates the "constitutive" endocytosis of transferrin
Abstract
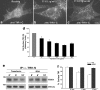
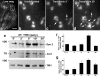
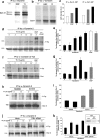
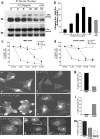
The mechanisms by which epithelial cells regulate clathrin-mediated endocytosis (CME) of transferrin are poorly defined and generally viewed as a constitutive process that occurs continuously without regulatory constraints. In this study, we demonstrate for the first time that endocytosis of the transferrin receptor is a regulated process that requires activated Src kinase and, subsequently, phosphorylation of two important components of the endocytic machinery, namely, the large GTPase dynamin 2 (Dyn2) and its associated actin-binding protein, cortactin (Cort). To our knowledge these findings are among the first to implicate an Src-mediated endocytic cascade in what was previously presumed to be a nonregulated internalization process.
Figures



References
-
- Ahn, S., J. Kim, C. L. Lucaveche, M. C. Reedy, L. M. Luttrell, R. J. Lefkowitz, and Y. Daaka. 2002. Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J. Biol. Chem. 277:26642-26651. - PubMed
-
- Ahn, S., S. Maudsley, L. M. Luttrell, R. J. Lefkowitz, and Y. Daaka. 1999. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem. 274:1185-1188. - PubMed
-
- Behrens, J., L. Vakaet, R. Friis, E. Winterhager, F. Van Roy, M. M. Mareel, and W. Birchmeier. 1993. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120:757-766. - PMC - PubMed
-
- Beutler, E., A. V. Hoffbrand, and J. D. Cook. 2003. Iron deficiency and overload. Hematology Am. Soc Hematol. Educ. Program 2003:40-61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
