Miltefosine efficiently eliminates Leishmania major amastigotes from infected murine dendritic cells without altering their immune functions
- PMID: 19995922
- PMCID: PMC2812123
- DOI: 10.1128/AAC.01014-09
Miltefosine efficiently eliminates Leishmania major amastigotes from infected murine dendritic cells without altering their immune functions
Abstract
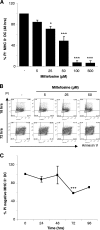
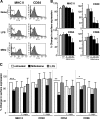
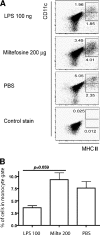
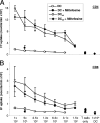
As a treatment for leishmaniasis, miltefosine exerts direct toxic effects on the parasites. Miltefosine also modulates immune cells such as macrophages, leading to parasite elimination via oxidative radicals. Dendritic cells (DC) are critical for initiation of protective immunity against Leishmania through induction of Th1 immunity via interleukin 12 (IL-12). Here, we investigated the effects of miltefosine on DC in Leishmania major infections. When cocultured with miltefosine for 4 days, the majority of in vitro-infected DC were free of parasites. Miltefosine treatment did not influence DC maturation (upregulation of major histocompatibility complex II [MHC II] or costimulatory molecules, e.g., CD40, CD54, and CD86) or significantly alter cytokine release (IL-12, tumor necrosis factor alpha [TNF-alpha], or IL-10). Further, miltefosine DC treatment did not alter antigen presentation, since unrestricted antigen-specific proliferation of CD4+ and CD8+ T cells was observed upon stimulation with miltefosine-treated, infected DC. In addition, miltefosine application in vivo did not lead to maturation/emigration of skin DC. DC NO- production, a mechanism used by phagocytes to rid themselves of intracellular parasites, was also unaltered upon miltefosine treatment. Our data confirm prior studies indicating that in contrast to, e.g., pentavalent antimonials, miltefosine functions independently of the immune system, mostly through direct toxicity against the Leishmania parasite.
Figures


Similar articles
-
Leishmania major-infected murine langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous Leishmaniasis.Eur J Immunol. 2000 Dec;30(12):3498-506. doi: 10.1002/1521-4141(2000012)30:12<3498::AID-IMMU3498>3.0.CO;2-6. Eur J Immunol. 2000. PMID: 11093169
-
Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity.J Exp Med. 1998 Oct 19;188(8):1547-52. doi: 10.1084/jem.188.8.1547. J Exp Med. 1998. PMID: 9782133 Free PMC article.
-
Investigation of anti-leishmanial efficacy of miltefosine and ketoconazole loaded on nanoniosomes.Acta Trop. 2018 Sep;185:69-76. doi: 10.1016/j.actatropica.2018.05.002. Epub 2018 May 4. Acta Trop. 2018. PMID: 29733808
-
Systematic Review of Host-Mediated Activity of Miltefosine in Leishmaniasis through Immunomodulation.Antimicrob Agents Chemother. 2019 Jun 24;63(7):e02507-18. doi: 10.1128/AAC.02507-18. Print 2019 Jul. Antimicrob Agents Chemother. 2019. PMID: 31036692 Free PMC article.
-
Miltefosine--discovery of the antileishmanial activity of phospholipid derivatives.Trans R Soc Trop Med Hyg. 2006 Dec;100 Suppl 1:S4-8. doi: 10.1016/j.trstmh.2006.03.009. Epub 2006 Aug 14. Trans R Soc Trop Med Hyg. 2006. PMID: 16904717 Review.
Cited by
-
Oral treatment with T6-loaded yeast cell wall particles reduces the parasitemia in murine visceral leishmaniasis model.Sci Rep. 2019 Dec 27;9(1):20080. doi: 10.1038/s41598-019-56647-w. Sci Rep. 2019. PMID: 31882925 Free PMC article.
-
Antiparasitic Activity of Fluorophenyl-Substituted Pyrimido[1,2-a]benzimidazoles.Biomedicines. 2023 Jan 14;11(1):219. doi: 10.3390/biomedicines11010219. Biomedicines. 2023. PMID: 36672727 Free PMC article.
-
Protective and pathologic immune responses in human tegumentary leishmaniasis.Front Immunol. 2012 Oct 4;3:301. doi: 10.3389/fimmu.2012.00301. eCollection 2012. Front Immunol. 2012. PMID: 23060880 Free PMC article.
-
Antiparasitic and Antifungal Activities of Cetyl-Maritima, a New N-Cetyl-Modified Maritima Derivative.Antibiotics (Basel). 2025 Mar 19;14(3):321. doi: 10.3390/antibiotics14030321. Antibiotics (Basel). 2025. PMID: 40149131 Free PMC article.
-
Immunomodulatory Therapy of Visceral Leishmaniasis in Human Immunodeficiency Virus-Coinfected Patients.Front Immunol. 2018 Jan 12;8:1943. doi: 10.3389/fimmu.2017.01943. eCollection 2017. Front Immunol. 2018. PMID: 29375567 Free PMC article. Review.
References
-
- Azzouz, S., M. Maache, R. G. Garcia, and A. Osuna. 2005. Leishmanicidal activity of edelfosine, miltefosine and ilmofosine. Basic Clin. Pharmacol. Toxicol. 96:60-65. - PubMed
-
- Beckers, T., R. Voegeli, and P. Hilgard. 1994. Molecular and cellular effects of hexadecylphosphocholine (miltefosine) in human myeloid leukaemic cell lines. Eur. J. Cancer 30:2143-2150. - PubMed
-
- Brandonisio, O., M. A. Panaro, M. Sisto, A. Acquafredda, L. Fumarola, D. Leogrande, and V. Mitolo. 2001. Nitric oxide production by Leishmania-infected macrophages and modulation by cytokines and prostaglandins. Parassitologia 43(Suppl. 1):1-6. - PubMed
-
- Brandonisio, O., R. Spinelli, and M. Pepe. 2004. Dendritic cells in Leishmania infection. Microbes Infect. 6:1402-1409. - PubMed
-
- Croft, S. L., R. A. Neal, W. Pendergast, and J. H. Chan. 1987. The activity of alkyl phosphorylcholines and related derivatives against Leishmania donovani. Biochem. Pharmacol. 36:2633-2636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

