High concentration of raltegravir in semen of HIV-infected men: results from a substudy of the EASIER-ANRS 138 trial
- PMID: 19995925
- PMCID: PMC2812174
- DOI: 10.1128/AAC.01261-09
High concentration of raltegravir in semen of HIV-infected men: results from a substudy of the EASIER-ANRS 138 trial
Abstract
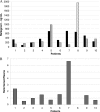
Raltegravir concentrations and human immunodeficiency virus type 1 (HIV-1) RNA levels in semen samples from 10 treatment-experienced HIV-1-infected patients were measured after 24 weeks of raltegravir-based highly active antiretroviral therapy (HAART). Semen and plasma HIV-1 RNA levels were below 100 copies/ml and 50 copies/ml, respectively, in all samples. The median raltegravir concentrations in semen samples (n=10) and in plasma samples (n=9) drawn simultaneously were 345 (range, 83 to 707) ng/ml and 206 (range, 106 to 986) ng/ml, respectively. The median semen-to-plasma ratio of raltegravir concentration was 1.42 (range, 0.52 to 6.66), indicating good although variable levels of drug penetration of raltegravir in the seminal compartment.
Figures
Similar articles
-
Lack of a Clinically Significant Pharmacokinetic Interaction between Etravirine and Raltegravir Using an Original Approach Based on Drug Metabolism, Protein Binding, and Penetration in Seminal Fluid: A Pharmacokinetic Substudy of the ANRS-163 ETRAL Study.Pharmacotherapy. 2019 Apr;39(4):514-520. doi: 10.1002/phar.2242. Epub 2019 Apr 1. Pharmacotherapy. 2019. PMID: 30815916 Clinical Trial.
-
Virological and immunological response in HIV-1-infected patients with multiple treatment failures receiving raltegravir and optimized background therapy, ANRS CO3 Aquitaine Cohort.J Antimicrob Chemother. 2009 Jun;63(6):1251-5. doi: 10.1093/jac/dkp114. Epub 2009 Mar 31. J Antimicrob Chemother. 2009. PMID: 19336453 Free PMC article. Clinical Trial.
-
Pharmacokinetics of maraviroc, raltegravir, darunavir, and etravirine in the semen of HIV-infected men.J Acquir Immune Defic Syndr. 2013 Feb 1;62(2):e58-60. doi: 10.1097/QAI.0b013e31827a0d71. J Acquir Immune Defic Syndr. 2013. PMID: 23328092 No abstract available.
-
Treatment of HIV infection with raltegravir.Expert Opin Pharmacother. 2009 May;10(7):1203-11. doi: 10.1517/14656560902911488. Expert Opin Pharmacother. 2009. PMID: 19385721 Review.
-
Raltegravir: in treatment-naive patients with HIV-1 infection.Drugs. 2010 Mar 26;70(5):631-42. doi: 10.2165/11204590-000000000-00000. Drugs. 2010. PMID: 20329808 Review.
Cited by
-
Anogenital HIV RNA in Thai men who have sex with men in Bangkok during acute HIV infection and after randomization to standard vs. intensified antiretroviral regimens.J Int AIDS Soc. 2015 May 7;18(1):19470. doi: 10.7448/IAS.18.1.19470. eCollection 2015. J Int AIDS Soc. 2015. PMID: 25956171 Free PMC article. Clinical Trial.
-
Raltegravir permeability across blood-tissue barriers and the potential role of drug efflux transporters.Antimicrob Agents Chemother. 2015 May;59(5):2572-82. doi: 10.1128/AAC.04594-14. Epub 2015 Feb 17. Antimicrob Agents Chemother. 2015. PMID: 25691630 Free PMC article.
-
The use of preexposure treatments for HIV prophylaxis.HIV AIDS (Auckl). 2012;4:17-28. doi: 10.2147/HIV.S25082. Epub 2012 Feb 3. HIV AIDS (Auckl). 2012. PMID: 22347807 Free PMC article.
-
A long-acting formulation of the integrase inhibitor raltegravir protects humanized BLT mice from repeated high-dose vaginal HIV challenges.J Antimicrob Chemother. 2016 Jun;71(6):1586-96. doi: 10.1093/jac/dkw042. Epub 2016 Mar 21. J Antimicrob Chemother. 2016. PMID: 27002074 Free PMC article.
-
Correlation between plasma, intracellular, and cervical tissue levels of raltegravir at steady-state dosing in healthy women.Antimicrob Agents Chemother. 2014 Jun;58(6):3360-5. doi: 10.1128/AAC.02757-13. Epub 2014 Mar 31. Antimicrob Agents Chemother. 2014. PMID: 24687503 Free PMC article.
References
-
- Cao, Y. J., and C. W. Hendrix. 2008. Male genital tract pharmacology: developments in quantitative methods to better understand a complex peripheral compartment. Clin. Pharmacol. Ther. 83:401-412. - PubMed
-
- Croxtall, J. D., and S. J. Keam. 2009. Raltegravir: a review of its use in the management of HIV infection in treatment-experienced patients. Drugs 69:1059-1075. - PubMed
-
- De Castro, N., J. Braun, I. Charreau, G. Pialoux, L. Cotte, C. Katlama, F. Raffi, L. Weiss, J. L. Meynard, Y. Yazdanpanah, C. Delaugerre, I. Madelaine-Chambrin, J. P. Aboulker, and J. M. Molina. 2009. Switch from enfuvirtide to raltegravir in virologically suppressed multidrug-resistant HIV-1-infected patients: a randomized open-label trial. Clin. Infect. Dis. 49:1259-1267. - PubMed
-
- Ghosn, J., M. L. Chaix, G. Peytavin, J. L. Bresson, J. Galimand, P. M. Girard, F. Raffi, I. Cohen-Codar, J. F. Delfraissy, and C. Rouzioux. 2008. Absence of HIV-1 shedding in male genital tract after 1 year of first-line lopinavir/ritonavir alone or in combination with zidovudine/lamivudine. J. Antimicrob. Chemother. 61:1344-1347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

