External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers
- PMID: 19995951
- PMCID: PMC2806471
- DOI: 10.1084/jem.20091527
External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers
Abstract
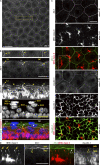
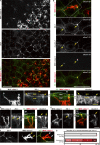
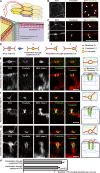
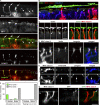
Outermost barriers are critical for terrestrial animals to avoid desiccation and to protect their bodies from foreign insults. Mammalian skin consists of two sets of barriers: stratum corneum (SC) and tight junctions (TJs). How acquisition of external antigens (Ags) by epidermal Langerhans cells (LCs) occur despite these barriers has remained unknown. We show that activation-induced LCs elongate their dendrites to penetrate keratinocyte (KC) TJs and survey the extra-TJ environment located outside of the TJ barrier, just beneath the SC. Penetrated dendrites uptake Ags from the tip where Ags colocalize with langerin/Birbeck granules. TJs at KC-KC contacts allow penetration of LC dendrites by dynamically forming new claudin-dependent bicellular- and tricellulin-dependent tricellular TJs at LC-KC contacts, thereby maintaining TJ integrity during Ag uptake. Thus, covertly under keratinized SC barriers, LCs and KCs demonstrate remarkable cooperation that enables LCs to gain access to external Ags that have violated the SC barrier while concomitantly retaining TJ barriers to protect intra-TJ environment.
Figures
Similar articles
-
[Dynamic cooperation between epidermal barriers and Langerhans cells].Nihon Rinsho Meneki Gakkai Kaishi. 2011;34(2):76-84. doi: 10.2177/jsci.34.76. Nihon Rinsho Meneki Gakkai Kaishi. 2011. PMID: 21628849 Review. Japanese.
-
Distinct behavior of human Langerhans cells and inflammatory dendritic epidermal cells at tight junctions in patients with atopic dermatitis.J Allergy Clin Immunol. 2014 Oct;134(4):856-64. doi: 10.1016/j.jaci.2014.08.001. J Allergy Clin Immunol. 2014. PMID: 25282566
-
[Structural and immunological barriers of the skin as potential therapeutic targets].Yakugaku Zasshi. 2014;134(5):623-7. doi: 10.1248/yakushi.14-00006-2. Yakugaku Zasshi. 2014. PMID: 24790044 Review. Japanese.
-
3D visualization of epidermal Langerhans cells.Methods Mol Biol. 2013;961:119-27. doi: 10.1007/978-1-62703-227-8_5. Methods Mol Biol. 2013. PMID: 23325638
-
Functional tight junction barrier localizes in the second layer of the stratum granulosum of human epidermis.J Dermatol Sci. 2013 Aug;71(2):89-99. doi: 10.1016/j.jdermsci.2013.04.021. Epub 2013 May 1. J Dermatol Sci. 2013. PMID: 23712060
Cited by
-
Regulation of tight junctions in upper airway epithelium.Biomed Res Int. 2013;2013:947072. doi: 10.1155/2013/947072. Epub 2012 Dec 29. Biomed Res Int. 2013. PMID: 23509817 Free PMC article. Review.
-
Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling.J Allergy Clin Immunol. 2012 Apr;129(4):1048-55.e6. doi: 10.1016/j.jaci.2012.01.063. Epub 2012 Mar 3. J Allergy Clin Immunol. 2012. PMID: 22385635 Free PMC article.
-
Topical calcineurin and mammalian target of rapamycin inhibitors in inflammatory dermatoses: Current challenges and nanotechnology‑based prospects (Review).Int J Mol Med. 2024 Oct;54(4):85. doi: 10.3892/ijmm.2024.5409. Epub 2024 Aug 12. Int J Mol Med. 2024. PMID: 39129316 Free PMC article. Review.
-
Inhibition of retinoid X receptor improved the morphology, localization of desmosomal proteins and paracellular permeability in three-dimensional cultures of mouse keratinocytes.Microscopy (Oxf). 2022 Jun 6;71(3):152-160. doi: 10.1093/jmicro/dfac007. Microscopy (Oxf). 2022. PMID: 35289919 Free PMC article.
-
Host-Microbe Interaction on the Skin and Its Role in the Pathogenesis and Treatment of Atopic Dermatitis.Pathogens. 2022 Jan 6;11(1):71. doi: 10.3390/pathogens11010071. Pathogens. 2022. PMID: 35056019 Free PMC article. Review.
References
-
- Aiba S., Katz S.I. 1990. Phenotypic and functional characteristics of in vivo-activated Langerhans cells. J. Immunol. 145:2791–2796 - PubMed
-
- Enk A.H., Angeloni V.L., Udey M.C., Katz S.I. 1993. An essential role for Langerhans cell-derived IL-1 beta in the initiation of primary immune responses in skin. J. Immunol. 150:3698–3704 - PubMed

