MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity
- PMID: 19995956
- PMCID: PMC2806469
- DOI: 10.1084/jem.20091333
MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity
Abstract
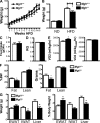
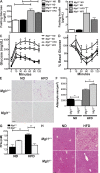
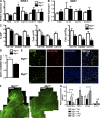
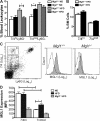
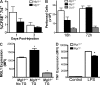
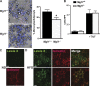
Adipose tissue macrophages (ATMs) play a critical role in obesity-induced inflammation and insulin resistance. Distinct subtypes of ATMs have been identified that differentially express macrophage galactose-type C-type lectin 1 (MGL1/CD301), a marker of alternatively activated macrophages. To evaluate if MGL1 is required for the anti-inflammatory function of resident (type 2) MGL1(+) ATMs, we examined the effects of diet-induced obesity (DIO) on inflammation and metabolism in Mgl1(-/-) mice. We found that Mgl1 is not required for the trafficking of type 2 ATMs to adipose tissue. Surprisingly, obese Mgl1(-/-) mice were protected from glucose intolerance, insulin resistance, and steatosis despite having more visceral fat. This protection was caused by a significant decrease in inflammatory (type 1) CD11c(+) ATMs in the visceral adipose tissue of Mgl1(-/-) mice. MGL1 was expressed specifically in 7/4(hi) inflammatory monocytes in the blood and obese Mgl1(-/-) mice had lower levels of 7/4(hi) monocytes. Mgl1(-/-) monocytes had decreased half-life after adoptive transfer and demonstrated decreased adhesion to adipocytes indicating a role for MGL1 in the regulation of monocyte function. This study identifies MGL1 as a novel regulator of inflammatory monocyte trafficking to adipose tissue in response to DIO.
Figures

Similar articles
-
Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes.Diabetes. 2008 Dec;57(12):3239-46. doi: 10.2337/db08-0872. Epub 2008 Oct 1. Diabetes. 2008. PMID: 18829989 Free PMC article.
-
Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance.Biochim Biophys Acta. 2014 Mar;1842(3):446-62. doi: 10.1016/j.bbadis.2013.05.017. Epub 2013 May 22. Biochim Biophys Acta. 2014. PMID: 23707515 Free PMC article. Review.
-
Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity.J Immunol. 2011 Aug 15;187(4):1942-9. doi: 10.4049/jimmunol.1100196. Epub 2011 Jul 8. J Immunol. 2011. PMID: 21742977 Free PMC article.
-
Increased macrophage migration into adipose tissue in obese mice.Diabetes. 2012 Feb;61(2):346-54. doi: 10.2337/db11-0860. Epub 2011 Dec 21. Diabetes. 2012. PMID: 22190646 Free PMC article.
-
Molecular mechanism of obesity-induced adipose tissue inflammation; the role of Mincle in adipose tissue fibrosis and ectopic lipid accumulation.Endocr J. 2020 Feb 28;67(2):107-111. doi: 10.1507/endocrj.EJ19-0417. Epub 2019 Dec 19. Endocr J. 2020. PMID: 31852849 Review.
Cited by
-
Monocyte Trafficking and Polarization Contribute to Sex Differences in Meta-Inflammation.Front Endocrinol (Lausanne). 2022 Mar 28;13:826320. doi: 10.3389/fendo.2022.826320. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35422759 Free PMC article.
-
Sphingosine kinase 1 regulates adipose proinflammatory responses and insulin resistance.Am J Physiol Endocrinol Metab. 2014 Apr 1;306(7):E756-68. doi: 10.1152/ajpendo.00549.2013. Epub 2014 Jan 28. Am J Physiol Endocrinol Metab. 2014. PMID: 24473437 Free PMC article.
-
Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation.PLoS One. 2013;8(3):e57929. doi: 10.1371/journal.pone.0057929. Epub 2013 Mar 5. PLoS One. 2013. PMID: 23472120 Free PMC article.
-
Haematopoietic leptin receptor deficiency does not affect macrophage accumulation in adipose tissue or systemic insulin sensitivity.J Endocrinol. 2012 Mar;212(3):343-51. doi: 10.1530/JOE-11-0338. Epub 2011 Dec 22. J Endocrinol. 2012. PMID: 22194312 Free PMC article.
-
S1P/S1PR3 signalling axis protects against obesity-induced metabolic dysfunction.Adipocyte. 2022 Dec;11(1):69-83. doi: 10.1080/21623945.2021.2021700. Adipocyte. 2022. PMID: 35094654 Free PMC article.
References
-
- Bouhlel M.A., Derudas B., Rigamonti E., Dièvart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., et al. 2007. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6:137–143 10.1016/j.cmet.2007.06.010 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

