Rapid beta-lactam-induced lysis requires successful assembly of the cell division machinery
- PMID: 19995973
- PMCID: PMC2799840
- DOI: 10.1073/pnas.0911674106
Rapid beta-lactam-induced lysis requires successful assembly of the cell division machinery
Abstract
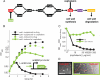
Beta-lactam antibiotics inhibit penicillin binding proteins (PBPs) involved in peptidoglycan synthesis. Although inhibition of peptidoglycan biosynthesis is generally thought to induce cell lysis, the pattern and mechanism of cell lysis can vary substantially. Beta-lactams that inhibit FtsI, the only division specific PBP, block cell division and result in growth as filaments. These filaments ultimately lyse through a poorly understood mechanism. Here we find that one such beta-lactam, cephalexin, can, under certain conditions, lead instead to rapid lysis at nascent division sites through a process that requires the complete and ordered assembly of the divisome, the essential machinery involved in cell division. We propose that this assembly process (in which the localization of cell wall hydrolases depends on properly targeted FtsN, which in turn depends on the presence of FtsI) ensures that the biosynthetic machinery to form new septa is in place before the machinery to degrade septated daughter cells is enabled. Beta-lactams that target FtsI subvert this mechanism by inhibiting FtsI without perturbing the normal assembly of the cell division machinery and the consequent activation of cell wall hydrolases. One seemingly paradoxical implication of our results is that beta-lactam therapy may be improved by promoting active cell division.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Inactivation of FtsI inhibits constriction of the FtsZ cytokinetic ring and delays the assembly of FtsZ rings at potential division sites.Proc Natl Acad Sci U S A. 1997 Jan 21;94(2):559-64. doi: 10.1073/pnas.94.2.559. Proc Natl Acad Sci U S A. 1997. PMID: 9012823 Free PMC article.
-
The ClpX chaperone controls autolytic splitting of Staphylococcus aureus daughter cells, but is bypassed by β-lactam antibiotics or inhibitors of WTA biosynthesis.PLoS Pathog. 2019 Sep 13;15(9):e1008044. doi: 10.1371/journal.ppat.1008044. eCollection 2019 Sep. PLoS Pathog. 2019. PMID: 31518377 Free PMC article.
-
Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery.Cell. 2014 Dec 4;159(6):1300-11. doi: 10.1016/j.cell.2014.11.017. Cell. 2014. PMID: 25480295 Free PMC article.
-
From penicillin-binding proteins to the lysis and death of bacteria: a 1979 view.Rev Infect Dis. 1979 May-Jun;1(3):434-67. doi: 10.1093/clinids/1.3.434. Rev Infect Dis. 1979. PMID: 45147 Review.
-
β-lactam resistance: The role of low molecular weight penicillin binding proteins, β-lactamases and ld-transpeptidases in bacteria associated with respiratory tract infections.IUBMB Life. 2018 Sep;70(9):855-868. doi: 10.1002/iub.1761. Epub 2018 May 2. IUBMB Life. 2018. PMID: 29717815 Review.
Cited by
-
Rapid cytometric antibiotic susceptibility testing utilizing adaptive multidimensional statistical metrics.Anal Chem. 2015 Feb 3;87(3):1941-9. doi: 10.1021/ac504241x. Epub 2015 Jan 13. Anal Chem. 2015. PMID: 25540985 Free PMC article.
-
Distinct single-cell morphological dynamics under beta-lactam antibiotics.Mol Cell. 2012 Dec 14;48(5):705-12. doi: 10.1016/j.molcel.2012.09.016. Epub 2012 Oct 25. Mol Cell. 2012. PMID: 23103254 Free PMC article.
-
Mutations Decreasing Intrinsic β-Lactam Resistance Are Linked to Cell Division in the Nosocomial Pathogen Acinetobacter baumannii.Antimicrob Agents Chemother. 2016 May 23;60(6):3751-8. doi: 10.1128/AAC.00361-16. Print 2016 Jun. Antimicrob Agents Chemother. 2016. PMID: 27067318 Free PMC article.
-
A switch in surface polymer biogenesis triggers growth-phase-dependent and antibiotic-induced bacteriolysis.Elife. 2019 Apr 9;8:e44912. doi: 10.7554/eLife.44912. Elife. 2019. PMID: 30964003 Free PMC article.
-
A conformational switch controls cell wall-remodelling enzymes required for bacterial cell division.Mol Microbiol. 2012 Aug;85(4):768-81. doi: 10.1111/j.1365-2958.2012.08138.x. Epub 2012 Jul 11. Mol Microbiol. 2012. PMID: 22715947 Free PMC article.
References
-
- Weidel W, Pelzer H. Bagshaped Macromolecules—a New Outlook on Bacterial Cell Walls. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. - PubMed
-
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: How the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. - PubMed
-
- Goehring NW, Beckwith J. Diverse paths to midcell: Assembly of the bacterial cell division machinery. Curr Biol. 2005;15:R514–R526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

