Fibrinolytic cross-talk: a new mechanism for plasmin formation
- PMID: 19996088
- PMCID: PMC2896557
- DOI: 10.1182/blood-2009-06-228817
Fibrinolytic cross-talk: a new mechanism for plasmin formation
Abstract
Fibrinolysis and pericellular proteolysis depend on molecular coassembly of plasminogen and its activator on cell, fibrin, or matrix surfaces. We report here the existence of a fibrinolytic cross-talk mechanism bypassing the requirement for their molecular coassembly on the same surface. First, we demonstrate that, despite impaired binding of Glu-plasminogen to the cell membrane by epsilon-aminocaproic acid (epsilon-ACA) or by a lysine-binding site-specific mAb, plasmin is unexpectedly formed by cell-associated urokinase (uPA). Second, we show that Glu-plasminogen bound to carboxy-terminal lysine residues in platelets, fibrin, or extracellular matrix components (fibronectin, laminin) is transformed into plasmin by uPA expressed on monocytes or endothelial cell-derived microparticles but not by tissue-type plasminogen activator (tPA) expressed on neurons. A 2-fold increase in plasmin formation was observed over activation on the same surface. Altogether, these data indicate that cellular uPA but not tPA expressed by distinct cells is specifically involved in the recognition of conformational changes and activation of Glu-plasminogen bound to other biologic surfaces via a lysine-dependent mechanism. This uPA-driven cross-talk mechanism generates plasmin in situ with a high efficiency, thus highlighting its potential physiologic relevance in fibrinolysis and matrix proteolysis induced by inflammatory cells or cell-derived microparticles.
Conflict of interest statement
Figures
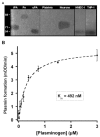
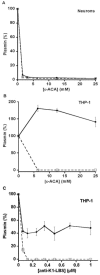
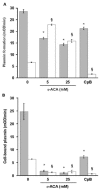
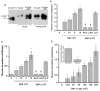
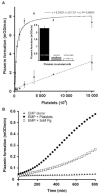
 (concentration equivalent to that bound to platelets), or with buffer (▲) in the presence of 0.75 mM CBS0065. The formation of plasmin (mOD/min) was detected by measuring the release of p-nitroaniline. Results are expressed as rate of plasmin formation (n = 3). A representative experiment is shown.
(concentration equivalent to that bound to platelets), or with buffer (▲) in the presence of 0.75 mM CBS0065. The formation of plasmin (mOD/min) was detected by measuring the release of p-nitroaniline. Results are expressed as rate of plasmin formation (n = 3). A representative experiment is shown.

Similar articles
-
Tranexamic acid mediates proinflammatory and anti-inflammatory signaling via complement C5a regulation in a plasminogen activator-dependent manner.J Trauma Acute Care Surg. 2019 Jan;86(1):101-107. doi: 10.1097/TA.0000000000002092. J Trauma Acute Care Surg. 2019. PMID: 30575685
-
Overview on fibrinolysis: plasminogen activation pathways on fibrin and cell surfaces.Chem Phys Lipids. 1994 Jan;67-68:353-62. doi: 10.1016/0009-3084(94)90157-0. Chem Phys Lipids. 1994. PMID: 8187235 Review.
-
The fibrinolytic system: A new target for treatment of depression with psychedelics.Med Hypotheses. 2017 Mar;100:46-53. doi: 10.1016/j.mehy.2017.01.013. Epub 2017 Jan 23. Med Hypotheses. 2017. PMID: 28236848
-
Differences between neonates and adults in the urokinase-plasminogen activator (u-PA) pathway of the fibrinolytic system.Thromb Res. 2000 Nov 15;100(4):341-51. doi: 10.1016/s0049-3848(00)00322-4. Thromb Res. 2000. PMID: 11113278
-
Fibrinolysis: the key to new pathogenetic mechanisms.Curr Med Chem. 2008;15(9):923-9. doi: 10.2174/092986708783955455. Curr Med Chem. 2008. PMID: 18473800 Review.
Cited by
-
In Vitro Study of the Fibrinolytic Activity via Single Chain Urokinase-Type Plasminogen Activator and Molecular Docking of FGFC1.Molecules. 2021 Mar 24;26(7):1816. doi: 10.3390/molecules26071816. Molecules. 2021. PMID: 33804930 Free PMC article.
-
Membrane microvesicles: a circulating source for fibrinolysis, new antithrombotic messengers.Haematologica. 2013 Jul;98(7):e75-6. doi: 10.3324/haematol.2013.088948. Haematologica. 2013. PMID: 23813650 Free PMC article. No abstract available.
-
Phosphatidylserine as an anchor for plasminogen and its plasminogen receptor, histone H2B, to the macrophage surface.J Thromb Haemost. 2011 Feb;9(2):339-49. doi: 10.1111/j.1538-7836.2010.04132.x. J Thromb Haemost. 2011. PMID: 21040449 Free PMC article.
-
Plasminogen receptors: the first quarter century.Semin Thromb Hemost. 2013 Jun;39(4):329-37. doi: 10.1055/s-0033-1334483. Epub 2013 Mar 26. Semin Thromb Hemost. 2013. PMID: 23532575 Free PMC article. Review.
-
New Insight on the Role of Plasminogen Receptor in Cancer Progression.Cancer Growth Metastasis. 2015 Jul 29;8:35-42. doi: 10.4137/CGM.S27335. eCollection 2015. Cancer Growth Metastasis. 2015. PMID: 26279629 Free PMC article. Review.
References
-
- Petersen TE, Martzen MR, Ichinose A, Davie EW. Characterization of the gene for human plasminogen, a key proenzyme in the fibrinolytic system. J Biol Chem. 1990 Apr 15;265(11):6104–6111. - PubMed
-
- Tulinsky A, Park CH, Mao B, Llinas M. Lysine/fibrin binding sites of kringles modeled after the structure of kringle 1 of prothrombin. Proteins. 1988;3(2):85–96. - PubMed
-
- Herren T, Swaisgood C, Plow EF. Regulation of plasminogen receptors. Front Biosci. 2003 Jan 1;8:d1–8. - PubMed
-
- Lucas MA, Fretto LJ, McKee PA. The binding of human plasminogen to fibrin and fibrinogen. J Biol Chem. 1983 Apr 10;258(7):4249–4256. - PubMed
-
- Knudsen BS, Silverstein RL, Leung LL, Harpel PC, Nachman RL. Binding of plasminogen to extracellular matrix. J Biol Chem. 1986 Aug 15;261(23):10765–10771. - PubMed

