Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance
- PMID: 19996103
- PMCID: PMC2825415
- DOI: 10.1074/jbc.M109.056648
Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance
Abstract
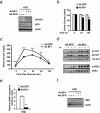
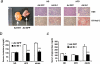
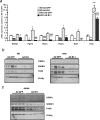
The unfolded protein response (UPR) or endoplasmic reticulum (ER) stress response is a physiological process enabling cells to cope with altered protein synthesis demands. However, under conditions of obesity, prolonged activation of the UPR has been shown to have deteriorating effects on different metabolic pathways. Here we identify Bax inhibitor-1 (BI-1), an evolutionary conserved ER-membrane protein, as a novel modulator of the obesity-associated alteration of the UPR. BI-1 partially inhibits the UPR by interacting with IRE1alpha and inhibiting IRE1alpha endonuclease activity as seen on the splicing of the transcription factor Xbp-1. Because we observed a down-regulation of BI-1 expression in liver and muscle of genetically obese ob/ob and db/db mice as well as in mice with diet-induced obesity in vivo, we investigated the effect of restoring BI-1 expression on metabolic processes in these mice. Importantly, BI-1 overexpression by adenoviral gene transfer dramatically improved glucose metabolism in both standard diet-fed mice as well as in mice with diet-induced obesity and, critically, reversed hyperglycemia in db/db mice. This improvement in whole body glucose metabolism and insulin sensitivity was due to dramatically reduced gluconeogenesis as shown by reduction of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase expression. Taken together, these results identify BI-1 as a critical regulator of ER stress responses in the development of obesity-associated insulin resistance and provide proof of concept evidence that gene transfer-mediated elevations in hepatic BI-1 may represent a promising approach for the treatment of type 2 diabetes.
Figures
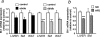



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

