Huntingtin-associated protein-1 interacts with pro-brain-derived neurotrophic factor and mediates its transport and release
- PMID: 19996106
- PMCID: PMC2820788
- DOI: 10.1074/jbc.M109.073197
Huntingtin-associated protein-1 interacts with pro-brain-derived neurotrophic factor and mediates its transport and release
Abstract
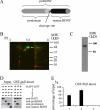
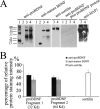
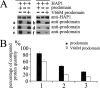
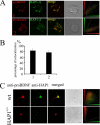
Brain-derived neurotrophic factor (BDNF) plays a pivotal role in brain development and synaptic plasticity. It is synthesized as a precursor (pro-BDNF), sorted into the secretory pathway, transported along dendrites and axons, and released in an activity-dependent manner. Mutant Huntingtin with expanded polyglutamine (polyQ) and the V66M polymorphism of BDNF reduce the dendritic distribution and axonal transport of BDNF. However, the mechanism underlying this defective transport remains unclear. Here, we report that Huntingtin-associated protein-1 (HAP1) interacts with the prodomain of BDNF and that the interaction was reduced in the presence of polyQ-expanded Huntingtin and BDNF V66M. Consistently, there was reduced coimmunoprecipitation of pro-BDNF with HAP1 in the brain homogenate of Huntington disease. Pro-BDNF distribution in the neuronal processes and its accumulation in the proximal and distal segments of crushed sciatic nerve and the activity-dependent release of pro-BDNF were abolished in HAP1(-/-) mice. These results suggest that HAP1 may participate in axonal transport and activity-dependent release of pro-BDNF by interacting with the BDNF prodomain. Accordingly, the decreased interaction between HAP1 and pro-BDNF in Huntington disease may reduce the release and transport of BDNF.
Figures


Similar articles
-
Precursor of brain-derived neurotrophic factor (proBDNF) forms a complex with Huntingtin-associated protein-1 (HAP1) and sortilin that modulates proBDNF trafficking, degradation, and processing.J Biol Chem. 2011 May 6;286(18):16272-84. doi: 10.1074/jbc.M110.195347. Epub 2011 Feb 28. J Biol Chem. 2011. PMID: 21357693 Free PMC article.
-
Mutant huntingtin impairs the post-Golgi trafficking of brain-derived neurotrophic factor but not its Val66Met polymorphism.J Neurosci. 2006 Dec 6;26(49):12748-57. doi: 10.1523/JNEUROSCI.3873-06.2006. J Neurosci. 2006. PMID: 17151278 Free PMC article.
-
Modification of Mecp2 dosage alters axonal transport through the Huntingtin/Hap1 pathway.Neurobiol Dis. 2012 Feb;45(2):786-95. doi: 10.1016/j.nbd.2011.11.002. Epub 2011 Nov 15. Neurobiol Dis. 2012. PMID: 22127389
-
[Huntington's disease: intracellular signaling pathways and neuronal death].J Soc Biol. 2005;199(3):247-51. doi: 10.1051/jbio:2005026. J Soc Biol. 2005. PMID: 16471265 Review. French.
-
Molecular aspects of Huntington's disease.J Neurosci Res. 1998 Nov 1;54(3):301-8. doi: 10.1002/(SICI)1097-4547(19981101)54:3<301::AID-JNR1>3.0.CO;2-W. J Neurosci Res. 1998. PMID: 9819135 Review.
Cited by
-
Perturbation of the Akt/Gsk3-β signalling pathway is common to Drosophila expressing expanded untranslated CAG, CUG and AUUCU repeat RNAs.Hum Mol Genet. 2011 Jul 15;20(14):2783-94. doi: 10.1093/hmg/ddr177. Epub 2011 Apr 25. Hum Mol Genet. 2011. PMID: 21518731 Free PMC article.
-
The pro-domains of neurotrophins, including BDNF, are linked to Alzheimer's disease through a toxic synergy with Aβ.Hum Mol Genet. 2015 Jul 15;24(14):3929-38. doi: 10.1093/hmg/ddv130. Epub 2015 May 7. Hum Mol Genet. 2015. PMID: 25954034 Free PMC article.
-
Differential Transcriptome Profiling Unveils Novel Deregulated Gene Signatures Involved in Pathogenesis of Alzheimer's Disease.Biomedicines. 2022 Mar 6;10(3):611. doi: 10.3390/biomedicines10030611. Biomedicines. 2022. PMID: 35327413 Free PMC article.
-
Precursor of brain-derived neurotrophic factor (proBDNF) forms a complex with Huntingtin-associated protein-1 (HAP1) and sortilin that modulates proBDNF trafficking, degradation, and processing.J Biol Chem. 2011 May 6;286(18):16272-84. doi: 10.1074/jbc.M110.195347. Epub 2011 Feb 28. J Biol Chem. 2011. PMID: 21357693 Free PMC article.
-
Integral Characterization of Defective BDNF/TrkB Signalling in Neurological and Psychiatric Disorders Leads the Way to New Therapies.Int J Mol Sci. 2017 Jan 28;18(2):268. doi: 10.3390/ijms18020268. Int J Mol Sci. 2017. PMID: 28134845 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

