Identification of human plasma proteins as major clients for the extracellular chaperone clusterin
- PMID: 19996109
- PMCID: PMC2823492
- DOI: 10.1074/jbc.M109.079566
Identification of human plasma proteins as major clients for the extracellular chaperone clusterin
Abstract
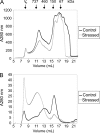
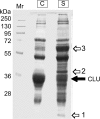
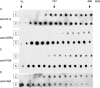
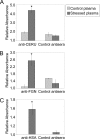
Clusterin (CLU) is an extracellular chaperone that is likely to play an important role in protein folding quality control. This study identified three deposition disease-associated proteins as major plasma clients for clusterin by studying CLU-client complexes formed in response to physiologically relevant stress (shear stress, approximately 36 dynes/cm(2) at 37 degrees C). Analysis of plasma samples by size exclusion chromatography indicated that (i) relative to control plasma, stressed plasma contained proportionally more soluble protein species of high molecular weight, and (ii) high molecular weight species were far more abundant when proteins purified by anti-CLU immunoaffinity chromatography from stressed plasma were compared with those purified from control plasma. SDS-PAGE and Western blot analyses indicated that a variety of proteins co-purified with CLU from both stressed and control plasma; however, several proteins were uniquely present or much more abundant when plasma was stressed. These proteins were identified by mass spectrometry as ceruloplasmin, fibrinogen, and albumin. Immunodot blot analysis of size exclusion chromatography fractionated plasma suggested that CLU-client complexes generated in situ are very large and may reach >or=4 x 10(7) Da. Lastly, sandwich enzyme-linked immunosorbent assay detected complexes containing CLU and ceruloplasmin, fibrinogen, or albumin in stressed but not control plasma. We have previously proposed that CLU-client complexes serve as vehicles to dispose of damaged misfolded extracellular proteins in vivo via receptor-mediated endocytosis. A better understanding of these mechanisms is likely to ultimately lead to the identification of new therapies for extracellular protein deposition disorders.
Figures


Similar articles
-
Structural characterization of clusterin-chaperone client protein complexes.J Biol Chem. 2009 Aug 14;284(33):21920-21927. doi: 10.1074/jbc.M109.033688. Epub 2009 Jun 17. J Biol Chem. 2009. PMID: 19535339 Free PMC article.
-
Clusterin facilitates in vivo clearance of extracellular misfolded proteins.Cell Mol Life Sci. 2011 Dec;68(23):3919-31. doi: 10.1007/s00018-011-0684-8. Epub 2011 Apr 20. Cell Mol Life Sci. 2011. PMID: 21505792 Free PMC article.
-
Fucosylated clusterin in semen promotes the uptake of stress-damaged proteins by dendritic cells via DC-SIGN.Hum Reprod. 2015 Jul;30(7):1545-56. doi: 10.1093/humrep/dev113. Epub 2015 May 23. Hum Reprod. 2015. PMID: 26003430
-
Chapter 6: The chaperone action of Clusterin and its putative role in quality control of extracellular protein folding.Adv Cancer Res. 2009;104:89-114. doi: 10.1016/S0065-230X(09)04006-8. Adv Cancer Res. 2009. PMID: 19878774 Review.
-
Therapeutic Potential of the Molecular Chaperone and Matrix Metalloproteinase Inhibitor Clusterin for Dry Eye.Int J Mol Sci. 2020 Dec 24;22(1):116. doi: 10.3390/ijms22010116. Int J Mol Sci. 2020. PMID: 33374364 Free PMC article. Review.
Cited by
-
Expression of metabolic, tissue remodeling, oxidative stress, and inflammatory pathways in mammary tissue during involution in lactating dairy cows.Bioinform Biol Insights. 2010 Sep 20;4:85-97. doi: 10.4137/bbi.s5850. Bioinform Biol Insights. 2010. PMID: 20981268 Free PMC article.
-
Human pregnancy zone protein stabilizes misfolded proteins including preeclampsia- and Alzheimer's-associated amyloid beta peptide.Proc Natl Acad Sci U S A. 2019 Mar 26;116(13):6101-6110. doi: 10.1073/pnas.1817298116. Epub 2019 Mar 8. Proc Natl Acad Sci U S A. 2019. PMID: 30850528 Free PMC article.
-
Hypochlorite-induced structural modifications enhance the chaperone activity of human α2-macroglobulin.Proc Natl Acad Sci U S A. 2014 May 20;111(20):E2081-90. doi: 10.1073/pnas.1403379111. Epub 2014 May 5. Proc Natl Acad Sci U S A. 2014. PMID: 24799681 Free PMC article.
-
Novel ACE2 protein interactions relevant to COVID-19 predicted by evolutionary rate correlations.PeerJ. 2021 Sep 15;9:e12159. doi: 10.7717/peerj.12159. eCollection 2021. PeerJ. 2021. PMID: 34616619 Free PMC article.
-
Plasma Proteomic Analysis in Morquio A Disease.Int J Mol Sci. 2021 Jun 7;22(11):6165. doi: 10.3390/ijms22116165. Int J Mol Sci. 2021. PMID: 34200496 Free PMC article.
References
-
- Carrell R. W., Lomas D. A. (1997) Lancet 350, 134–138 - PubMed
-
- Carrell R. W., Gooptu B. (1998) Curr. Opin. Struct. Biol. 8, 799–809 - PubMed
-
- Thomas P. J., Qu B. H., Pedersen P. L. (1995) Trends Biochem. Sci. 20, 456–459 - PubMed
-
- Soto C. (2001) FEBS Lett. 498, 204–207 - PubMed
-
- Mullins R. F., Russell S. R., Anderson D. H., Hageman G. S. (2000) FASEB J. 14, 835–846 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

