Differential effects of leptin receptor mutation on male and female BBDR Gimap5-/Gimap5- spontaneously diabetic rats
- PMID: 19996157
- PMCID: PMC2841494
- DOI: 10.1152/physiolgenomics.00186.2009
Differential effects of leptin receptor mutation on male and female BBDR Gimap5-/Gimap5- spontaneously diabetic rats
Abstract
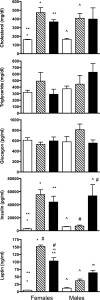
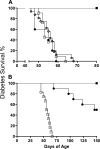
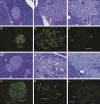
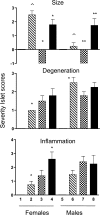
Rodents homozygous for autosomal leptin receptor gene mutations not only become obese, insulin resistant, and hyperleptinemic but also develop a dysregulated immune system. Using marker-assisted breeding to introgress the Koletsky rat leptin receptor mutant (lepr-/lepr-), we developed a novel congenic BBDR.(lepr-/lepr-) rat line to study the development of obesity and type 2 diabetes (T2D) in the BioBreeding (BB) diabetes-resistant (DR) rat. While heterozygous lepr (-/+) or homozygous (+/+) BBDR rats remained lean and metabolically normal, at 3 wk of age all BBDR.(lepr-/lepr-) rats were obese without hyperglycemia. Between 45 and 70 days of age, male but not female obese rats developed T2D. We had previously developed congenic BBDR.(Gimap5-/Gimap5-) rats, which carry an autosomal frameshift mutation in the Gimap5 gene linked to lymphopenia and spontaneous development of type 1 diabetes (T1D) without sex differences. Because the autoimmune-mediated destruction of pancreatic islet beta-cells may be affected not only by obesity but also by the absence of leptin receptor signaling, we next generated BBDR.(lepr-/lepr-,Gimap5-/Gimap5-) double congenic rats carrying the mutation for Gimap5 and T1D as well as the Lepr mutation for obesity and T2D. The hyperleptinemia rescued end-stage islets in BBDR.(lepr-/lepr-,Gimap5-/Gimap5-) congenic rats and induced an increase in islet size in both sexes, while T1D development was delayed and reduced only in females. These results demonstrate that obesity and T2D induced by introgression of the Koletsky leptin receptor mutation in the BBDR rat result in islet expansion associated with protection from T1D in female but not male BBDR.(lepr-/lepr-,Gimap5-/Gimap5-) congenic rats. BBDR.(lepr-/lepr-,Gimap5-/Gimap5-) congenic rats should prove valuable to study interactions between lack of leptin receptor signaling, obesity, and sex-specific T2D and T1D.
Figures


Similar articles
-
Genetic dissection reveals diabetes loci proximal to the gimap5 lymphopenia gene.Physiol Genomics. 2009 Jun 10;38(1):89-97. doi: 10.1152/physiolgenomics.00015.2009. Epub 2009 Apr 7. Physiol Genomics. 2009. PMID: 19351909 Free PMC article.
-
Characterization of a novel congenic strain of diabetic fatty (WBN/Kob-Lepr(fa)) rat.Biochem Biophys Res Commun. 2008 Feb 8;366(2):556-62. doi: 10.1016/j.bbrc.2007.12.003. Epub 2007 Dec 7. Biochem Biophys Res Commun. 2008. PMID: 18068663
-
Introgression of F344 rat genomic DNA on BB rat chromosome 4 generates diabetes-resistant lymphopenic BB rats.Diabetes. 2006 Dec;55(12):3351-7. doi: 10.2337/db06-0715. Diabetes. 2006. PMID: 17130479
-
The BBZDR/Wor rat model for investigating the complications of type 2 diabetes mellitus.ILAR J. 2004;45(3):292-302. doi: 10.1093/ilar.45.3.292. ILAR J. 2004. PMID: 15229376 Review.
-
Leptin receptor modulation of adiposity and fertility.Trends Endocrinol Metab. 2010 Jan;21(1):10-6. doi: 10.1016/j.tem.2009.07.004. Epub 2009 Oct 23. Trends Endocrinol Metab. 2010. PMID: 19854659 Free PMC article. Review.
Cited by
-
Sustained glucagon-like peptide 1 expression from encapsulated transduced cells to treat obese diabetic rats.J Biosci Bioeng. 2011 Apr;111(4):383-7. doi: 10.1016/j.jbiosc.2010.12.008. Epub 2011 Jan 8. J Biosci Bioeng. 2011. PMID: 21216666 Free PMC article.
-
Rat Models of Metabolic Syndrome.Methods Mol Biol. 2019;2018:269-285. doi: 10.1007/978-1-4939-9581-3_13. Methods Mol Biol. 2019. PMID: 31228162 Free PMC article.
-
Effect of exogenous leptin on serum levels of lipids, glucose, renal and hepatic variables in both genders of obese and streptozotocin-induced diabetic rats.Iran J Basic Med Sci. 2015 Nov;18(11):1072-8. Iran J Basic Med Sci. 2015. PMID: 26949493 Free PMC article.
-
Obesity and sex interact in the regulation of Alzheimer's disease.Neurosci Biobehav Rev. 2016 Aug;67:102-18. doi: 10.1016/j.neubiorev.2015.08.021. Epub 2015 Dec 18. Neurosci Biobehav Rev. 2016. PMID: 26708713 Free PMC article. Review.
-
Prolonged survival and improved glycemia in BioBreeding diabetic rats after early sustained exposure to glucagon-like peptide 1.J Gene Med. 2010 Jun;12(6):538-44. doi: 10.1002/jgm.1466. J Gene Med. 2010. PMID: 20527046 Free PMC article.
References
-
- Bieg S, Koike G, Jiang J, Klaff L, Pettersson A, MacMurray AJ, Jacob HJ, Lander ES, Lernmark A. Genetic isolation of iddm 1 on chromosome 4 in the BioBreeding (BB) rat. Mamm Genome 9:324–326, 1998 - PubMed
-
- Busso N, So A, Chobaz-Peclat V, Morard C, Martinez-Soria E, Talabot-Ayer D, Gabay C. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol 168:875–882, 2002 - PubMed
-
- Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271:994–996, 1996 - PubMed
-
- Dalberg U, Markholst H, Hornum L. Both Gimap5 and the diabetogenic BBDP allele of Gimap5 induce apoptosis in T cells. Int Immunol 19:447–453, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

