Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production
- PMID: 19996179
- PMCID: PMC2799849
- DOI: 10.1073/pnas.0911472106
Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production
Abstract
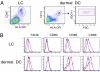
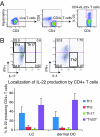
IL-22 is a cytokine that acts mainly on epithelial cells. In the skin, it mediates keratinocyte proliferation and epidermal hyperplasia and is thought to play a central role in inflammatory diseases with marked epidermal acanthosis, such as psoriasis. Although IL-22 was initially considered a Th17 cytokine, increasing evidence suggests that T helper cells can produce IL-22 even without IL-17 expression. In addition, we have shown the existence of this unique IL-22-producing T cell in normal skin and in the skin of psoriasis and atopic dermatitis patients. In the present study, we investigated the ability of cutaneous resident dendritic cells (DCs) to differentiate IL-22-producing cells. Using FACS, we isolated Langerhans cells (LCs; HLA-DR(+)CD207(+) cells) and dermal DCs (HLA-DR(hi)CD11c(+)BDCA-1(+) cells) from normal human epidermis and dermis, respectively. Both LCs and dermal DCs significantly induced IL-22-producing CD4(+) and CD8(+) T cells from peripheral blood T cells and naive CD4(+) T cells in mixed leukocyte reactions. LCs were more powerful in the induction of IL-22-producing cells than dermal DCs. Moreover, in vitro-generated LC-type DCs induced IL-22-producing cells more efficiently than monocyte-derived DCs. The induced IL-22 production was more correlated with IFN-gamma than IL-17. Surprisingly, the majority of IL-22-producing cells induced by LCs and dermal DCs lacked the expression of IL-17, IFN-gamma, and IL-4. Thus, LCs and dermal DCs preferentially induced helper T cells to produce only IL-22, possibly "Th22" cells. Our data indicate that cutaneous DCs, especially LCs, may control the generation of distinct IL-22 producing Th22 cells infiltrating into the skin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


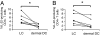
Similar articles
-
Differential capacity of human skin dendritic cells to polarize CD4+ T cells into IL-17, IL-21 and IL-22 producing cells.PLoS One. 2012;7(11):e45680. doi: 10.1371/journal.pone.0045680. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226194 Free PMC article.
-
Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells.J Invest Dermatol. 2009 Jan;129(1):79-88. doi: 10.1038/jid.2008.194. Epub 2008 Jul 17. J Invest Dermatol. 2009. PMID: 18633443 Free PMC article.
-
Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen-presenting cells that induce proallergic T cells.J Allergy Clin Immunol. 2007 Apr;119(4):982-90. doi: 10.1016/j.jaci.2007.01.003. Epub 2007 Feb 22. J Allergy Clin Immunol. 2007. PMID: 17320941
-
Psoriasis and other Th17-mediated skin diseases.J UOEH. 2010 Dec 1;32(4):317-28. doi: 10.7888/juoeh.32.317. J UOEH. 2010. PMID: 21226422 Review.
-
[Role of IL-22 in the pathogenesis of skin diseases].Nihon Rinsho Meneki Gakkai Kaishi. 2012;35(3):168-75. doi: 10.2177/jsci.35.168. Nihon Rinsho Meneki Gakkai Kaishi. 2012. PMID: 22790576 Review. Japanese.
Cited by
-
(Not) Home alone: Antigen presenting cell - T Cell communication in barrier tissues.Front Immunol. 2022 Sep 29;13:984356. doi: 10.3389/fimmu.2022.984356. eCollection 2022. Front Immunol. 2022. PMID: 36248804 Free PMC article. Review.
-
Interleukin 22 inhibits intracellular growth of Mycobacterium tuberculosis by enhancing calgranulin A expression.J Infect Dis. 2014 Feb 15;209(4):578-87. doi: 10.1093/infdis/jit495. Epub 2013 Sep 16. J Infect Dis. 2014. PMID: 24041785 Free PMC article.
-
Human Dendritic Cell Subsets, Ontogeny, and Impact on HIV Infection.Front Immunol. 2019 May 16;10:1088. doi: 10.3389/fimmu.2019.01088. eCollection 2019. Front Immunol. 2019. PMID: 31156637 Free PMC article. Review.
-
The pathophysiological role of dendritic cell subsets in psoriasis.BMB Rep. 2014 Feb;47(2):60-8. doi: 10.5483/bmbrep.2014.47.2.014. BMB Rep. 2014. PMID: 24411465 Free PMC article. Review.
-
Dendritic Cell Plasticity in Tumor-Conditioned Skin: CD14(+) Cells at the Cross-Roads of Immune Activation and Suppression.Front Immunol. 2013 Nov 25;4:403. doi: 10.3389/fimmu.2013.00403. Front Immunol. 2013. PMID: 24324467 Free PMC article. Review.
References
-
- Zenewicz LA, Flavell RA. IL-22 and inflammation: Leukin' through a glass onion. Eur J Immunol. 2008;38:3265–3268. - PubMed
-
- Gurney AL. IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int Immunopharmacol. 2004;4:669–677. - PubMed
-
- Wolk K, Kunz S, Asadullah K, Sabat R. Immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

