Integrated Molecular and Clinical Analysis of AKT Activation in Metastatic Melanoma
- PMID: 19996208
- PMCID: PMC2805170
- DOI: 10.1158/1078-0432.CCR-09-1985
Integrated Molecular and Clinical Analysis of AKT Activation in Metastatic Melanoma
Abstract
PURPOSE: Activation of the phosphoinositide 3-kinase (PI3K)-AKT pathway has been implicated in melanoma based primarily on the prevalence of mutations in PTEN and NRAS. To improve our understanding of the regulation and clinical significance of the PI3K-AKT pathway in melanoma, we quantitatively measured the levels of phosphorylated AKT, its substrate GSK3alpha/beta, and its negative regulator PTEN in clinical metastases. Results were compared with mutational status, clinical outcomes, and sites of metastasis. EXPERIMENTAL DESIGN: DNA and protein were isolated from dissected frozen melanoma metastases (n = 96). Activating mutations of BRAF, NRAS, AKT, PIK3CA, and KIT were detected by mass spectroscopy genotyping. Phosphorylated AKT (Ser473 and Thr308), P-GSK3alpha/beta, and PTEN protein expression were measured by reverse-phase protein array. A panel of human melanoma cells lines (n = 58) was analyzed for comparison. RESULTS: BRAF-mutant tumors had higher levels of P-AKT-Ser473 (P = 0.01), P-AKT-Thr308 (P = 0.002), and P-GSK3alpha/beta (P = 0.08) than NRAS-mutant tumors. Analysis of individual tumors showed that almost all tumors with elevated P-AKT had low PTEN levels; NRAS-mutant tumors had normal PTEN and lower P-AKT. Similar results were observed in melanoma cell lines. Stage III melanoma patients did not differ in overall survival based on activation status of the PI3K-AKT pathway. Brain metastases had significantly higher P-AKT and lower PTEN than lung or liver metastases. CONCLUSIONS: Quantitative interrogation of the PI3K-AKT pathway in melanoma reveals unexpected significant differences in AKT activation by NRAS mutation and PTEN loss, and hyperactivation of AKT in brain metastases. These findings have implications for the rational development of targeted therapy for this disease. (Clin Cancer Res 2009;15(24):7538-46).
Figures

 ), AKT3(
), AKT3( ), and KIT(△) mutations are indicated. C, Expression of P-AKT-Ser473. D, Expression of P-GSK3α/β.
), and KIT(△) mutations are indicated. C, Expression of P-AKT-Ser473. D, Expression of P-GSK3α/β.
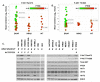
 ) and KIT(△) mutations are indicated. B, Levels of P-AKT-Thr308. C, Western blotting of human melanoma cells grown in serum-replete medium. Mutations in BRAF (“B”), NRAS (“N”), and KIT (“K”) are indicated, as are secondary alterations in the AKT pathway (“A”, AKT3-E17K; “P”, loss of PTEN expression). The same cell lysate (WM46) was run in the first lane of the first gel and the last lane of the second gel to ensure comparable immunoblot results. Loading was confirmed by ERK2 staining.
) and KIT(△) mutations are indicated. B, Levels of P-AKT-Thr308. C, Western blotting of human melanoma cells grown in serum-replete medium. Mutations in BRAF (“B”), NRAS (“N”), and KIT (“K”) are indicated, as are secondary alterations in the AKT pathway (“A”, AKT3-E17K; “P”, loss of PTEN expression). The same cell lysate (WM46) was run in the first lane of the first gel and the last lane of the second gel to ensure comparable immunoblot results. Loading was confirmed by ERK2 staining.

References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009 caac.20006. - PubMed
-
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. - PubMed
-
- Hocker T, Tsao H. Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants. Hum Mutat. 2007;28:578–88. - PubMed
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

