Inhibition of Functional Hyaluronan-CD44 Interactions in CD133-positive Primary Human Ovarian Carcinoma Cells by Small Hyaluronan Oligosaccharides
- PMID: 19996211
- PMCID: PMC2794991
- DOI: 10.1158/1078-0432.CCR-09-2317
Inhibition of Functional Hyaluronan-CD44 Interactions in CD133-positive Primary Human Ovarian Carcinoma Cells by Small Hyaluronan Oligosaccharides
Abstract
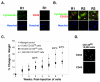
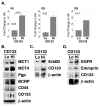
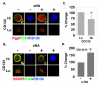
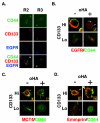
PURPOSE: CD44 is one of the most common markers used for identification of highly tumorigenic subpopulations of human carcinoma cells, but little is known about the function of CD44 or its major ligand, hyaluronan, in these cells. The purpose of this study was to investigate the involvement of hyaluronan and its interaction with CD44 in the properties of a tumorigenic subpopulation of primary ovarian carcinoma cells. EXPERIMENTAL DESIGN: A tumorigenic subpopulation was identified in ascites fluids from ovarian carcinoma patients by expression of high CD133 levels. Treatment with small hyaluronan oligosaccharides, which dissociate constitutive hyaluronan polymer-CD44 interactions, was used to test the importance of hyaluronan-CD44 interaction in assembly of multidrug and monocarboxylate transporters and receptor tyrosine kinases in the plasma membrane of cells with high CD133 levels, and in the tumorigenic capacity of the CD133-high subpopulation. RESULTS: Although total CD44 levels were similar in cells with high or low CD133 expression, CD44 was present in close association with transporters, receptor tyrosine kinases, and emmprin (CD147) in the plasma membrane of cells with high CD133 levels. Treatment with small hyaluronan oligosaccharides reduced association of the transporters and receptor tyrosine kinases with CD44 in the plasma membrane, diminished drug transporter activity, and inhibited i.p. tumorigenesis in these cells. CONCLUSIONS: We conclude that hyaluronan-CD44 interaction plays an important role in the properties of highly tumorigenic cells by stabilizing oncogenic complexes in their plasma membrane, and that treatment with hyaluronan-CD44 antagonists provides a logical therapeutic approach for abrogating the properties of these cells. (Clin Cancer Res 2009;15(24):7593-601).
Figures

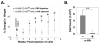
References
-
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. - PubMed
-
- Landen CN, Jr., Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. - PubMed
-
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. - PubMed
-
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

