Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer
- PMID: 19996287
- PMCID: PMC2805053
- DOI: 10.1158/0008-5472.CAN-09-1882
Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer
Abstract
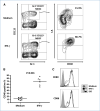
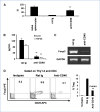
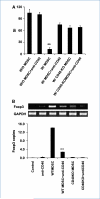
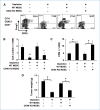
Immune tolerance to tumors is often associated with accumulation of myeloid-derived suppressor cells (MDSC) and an increase in the number of T-regulatory cells (Treg). In tumor-bearing mice, MDSCs can themselves facilitate the generation of tumor-specific Tregs. In this study, we demonstrate that expression of the immune stimulatory receptor CD40 on MDSCs is required to induce T-cell tolerance and Treg accumulation. In an immune reconstitution model, adoptive transfer of Gr-1+CD115+ monocytic MDSCs derived from CD40-deficient mice failed to recapitulate the ability of wild-type MDSCs to induce tolerance and Treg development in vivo. Agonistic anti-CD40 antibodies phenocopied the effect of CD40 deficiency and also improved the therapeutic efficacy of IL-12 and 4-1BB immunotherapy in the treatment of advanced tumors. Our findings suggest that CD40 is essential not only for MDSC-mediated immune suppression but also for tumor-specific Treg expansion. Blockade of CD40-CD40L interaction between MDSC and Treg may provide a new strategy to ablate tumoral immune suppression and thereby heighten responses to immunotherapy.
Figures


References
-
- Li Q, Pan PY, Gu P, Xu D, Chen SH. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 2004;64:1130–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

