Cell-cell contact formation governs Ca2+ signaling by TRPC4 in the vascular endothelium: evidence for a regulatory TRPC4-beta-catenin interaction
- PMID: 19996314
- PMCID: PMC2823560
- DOI: 10.1074/jbc.M109.060301
Cell-cell contact formation governs Ca2+ signaling by TRPC4 in the vascular endothelium: evidence for a regulatory TRPC4-beta-catenin interaction
Abstract
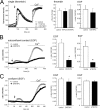
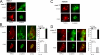
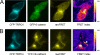
TRPC4 is well recognized as a prominent cation channel in the vascular endothelium, but its contribution to agonist-induced endothelial Ca(2+) entry is still a matter of controversy. Here we report that the cellular targeting and Ca(2+) signaling function of TRPC4 is determined by the state of cell-cell adhesions during endothelial phenotype transitions. TRPC4 surface expression in human microvascular endothelial cells (HMEC-1) increased with the formation of cell-cell contacts. Epidermal growth factor recruited TRPC4 into the plasma membrane of proliferating cells but initiated retrieval of TRPC4 from the plasma membrane in quiescent, barrier-forming cells. Epidermal growth factor-induced Ca(2+) entry was strongly promoted by the formation of cell-cell contacts, and both siRNA and dominant negative knockdown experiments revealed that TRPC4 mediates stimulated Ca(2+) entry exclusively in proliferating clusters that form immature cell-cell contacts. TRPC4 co-precipitated with the junctional proteins beta-catenin and VE-cadherin. Analysis of cellular localization of fluorescent fusion proteins provided further evidence for recruitment of TRPC4 into junctional complexes. Analysis of TRPC4 function in the HEK293 expression system identified beta-catenin as a signaling molecule that enables cell-cell contact-dependent promotion of TRPC4 function. Our results place TRPC4 as a Ca(2+) entry channel that is regulated by cell-cell contact formation and interaction with beta-catenin. TRPC4 is suggested to serve stimulated Ca(2+) entry in a specific endothelial state during the transition from a proliferating to a quiescent phenotype. Thus, TRPC4 may adopt divergent, as yet unappreciated functions in endothelial Ca(2+) homeostasis and emerges as a potential key player in endothelial phenotype switching and tuning of cellular growth factor signaling.
Figures



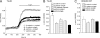

Similar articles
-
Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/β-catenin signaling.Stem Cells Dev. 2011 Jan;20(1):89-101. doi: 10.1089/scd.2010.0013. Epub 2010 Oct 18. Stem Cells Dev. 2011. PMID: 20446815 Free PMC article.
-
TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells.J Biol Chem. 2006 May 12;281(19):13588-13595. doi: 10.1074/jbc.M512205200. Epub 2006 Mar 13. J Biol Chem. 2006. PMID: 16537542
-
Close spatio-association of the transient receptor potential canonical 4 (TRPC4) channel with Gαi in TRPC4 activation process.Am J Physiol Cell Physiol. 2015 Jun 1;308(11):C879-89. doi: 10.1152/ajpcell.00374.2014. Epub 2015 Mar 18. Am J Physiol Cell Physiol. 2015. PMID: 25788576
-
Role of Ca2+ signaling in the regulation of endothelial permeability.Vascul Pharmacol. 2002 Nov;39(4-5):173-85. doi: 10.1016/s1537-1891(03)00007-7. Vascul Pharmacol. 2002. PMID: 12747958 Review.
-
TRPC4- and TRPC4-containing channels.Handb Exp Pharmacol. 2014;222:85-128. doi: 10.1007/978-3-642-54215-2_5. Handb Exp Pharmacol. 2014. PMID: 24756704 Review.
Cited by
-
Polarized Proteins in Endothelium and Their Contribution to Function.J Vasc Res. 2021;58(2):65-91. doi: 10.1159/000512618. Epub 2021 Jan 27. J Vasc Res. 2021. PMID: 33503620 Free PMC article. Review.
-
Regulation and function of the two-pore-domain (K2P) potassium channel Trek-1 in alveolar epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2012 Jan 1;302(1):L93-L102. doi: 10.1152/ajplung.00078.2011. Epub 2011 Sep 23. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 21949155 Free PMC article.
-
Significant contribution of TRPC6 channel-mediated Ca2+ influx to the pathogenesis of Crohn's disease fibrotic stenosis.J Smooth Muscle Res. 2016;52(0):78-92. doi: 10.1540/jsmr.52.78. J Smooth Muscle Res. 2016. PMID: 27818466 Free PMC article. Review.
-
Orai1 calcium channels in the vasculature.Pflugers Arch. 2012 Apr;463(5):635-47. doi: 10.1007/s00424-012-1090-2. Epub 2012 Mar 9. Pflugers Arch. 2012. PMID: 22402985 Free PMC article. Review.
-
Update on vascular endothelial Ca(2+) signalling: A tale of ion channels, pumps and transporters.World J Biol Chem. 2012 Jul 26;3(7):127-58. doi: 10.4331/wjbc.v3.i7.127. World J Biol Chem. 2012. PMID: 22905291 Free PMC article.
References
-
- Wakabayashi I., Poteser M., Groschner K. (2006) J. Vasc. Res. 43, 238–250 - PubMed
-
- Freichel M., Suh S. H., Pfeifer A., Schweig U., Trost C., Weissgerber P., Biel M., Philipp S., Freise D., Droogmans G., Hofmann F., Flockerzi V., Nilius B. (2001) Nat. Cell Biol. 3, 121–127 - PubMed
-
- Tiruppathi C., Freichel M., Vogel S. M., Paria B. C., Mehta D., Flockerzi V., Malik A. B. (2002) Circ. Res. 91, 70–76 - PubMed
-
- Cioffi D. L., Stevens T. (2006) Microcirculation 13, 709–723 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

