Cell-cell contact formation governs Ca2+ signaling by TRPC4 in the vascular endothelium: evidence for a regulatory TRPC4-beta-catenin interaction
- PMID: 19996314
- PMCID: PMC2823560
- DOI: 10.1074/jbc.M109.060301
Cell-cell contact formation governs Ca2+ signaling by TRPC4 in the vascular endothelium: evidence for a regulatory TRPC4-beta-catenin interaction
Abstract
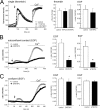
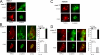
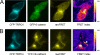
TRPC4 is well recognized as a prominent cation channel in the vascular endothelium, but its contribution to agonist-induced endothelial Ca(2+) entry is still a matter of controversy. Here we report that the cellular targeting and Ca(2+) signaling function of TRPC4 is determined by the state of cell-cell adhesions during endothelial phenotype transitions. TRPC4 surface expression in human microvascular endothelial cells (HMEC-1) increased with the formation of cell-cell contacts. Epidermal growth factor recruited TRPC4 into the plasma membrane of proliferating cells but initiated retrieval of TRPC4 from the plasma membrane in quiescent, barrier-forming cells. Epidermal growth factor-induced Ca(2+) entry was strongly promoted by the formation of cell-cell contacts, and both siRNA and dominant negative knockdown experiments revealed that TRPC4 mediates stimulated Ca(2+) entry exclusively in proliferating clusters that form immature cell-cell contacts. TRPC4 co-precipitated with the junctional proteins beta-catenin and VE-cadherin. Analysis of cellular localization of fluorescent fusion proteins provided further evidence for recruitment of TRPC4 into junctional complexes. Analysis of TRPC4 function in the HEK293 expression system identified beta-catenin as a signaling molecule that enables cell-cell contact-dependent promotion of TRPC4 function. Our results place TRPC4 as a Ca(2+) entry channel that is regulated by cell-cell contact formation and interaction with beta-catenin. TRPC4 is suggested to serve stimulated Ca(2+) entry in a specific endothelial state during the transition from a proliferating to a quiescent phenotype. Thus, TRPC4 may adopt divergent, as yet unappreciated functions in endothelial Ca(2+) homeostasis and emerges as a potential key player in endothelial phenotype switching and tuning of cellular growth factor signaling.
Figures



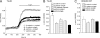

References
-
- Wakabayashi I., Poteser M., Groschner K. (2006) J. Vasc. Res. 43, 238–250 - PubMed
-
- Freichel M., Suh S. H., Pfeifer A., Schweig U., Trost C., Weissgerber P., Biel M., Philipp S., Freise D., Droogmans G., Hofmann F., Flockerzi V., Nilius B. (2001) Nat. Cell Biol. 3, 121–127 - PubMed
-
- Tiruppathi C., Freichel M., Vogel S. M., Paria B. C., Mehta D., Flockerzi V., Malik A. B. (2002) Circ. Res. 91, 70–76 - PubMed
-
- Cioffi D. L., Stevens T. (2006) Microcirculation 13, 709–723 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

