Structural basis for the recognition of oxidized phospholipids in oxidized low density lipoproteins by class B scavenger receptors CD36 and SR-BI
- PMID: 19996318
- PMCID: PMC2836050
- DOI: 10.1074/jbc.M109.082800
Structural basis for the recognition of oxidized phospholipids in oxidized low density lipoproteins by class B scavenger receptors CD36 and SR-BI
Abstract
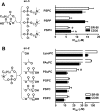
Specific oxidized phospholipids (oxPC(CD36)) accumulate in vivo at sites of oxidative stress and serve as high affinity ligands for scavenger receptors class B (CD36 and SR-BI). Recognition of oxPC(CD36) by scavenger receptors plays a role in several pathophysiological processes. The structural basis for the recognition of oxPC(CD36) by CD36 and SR-BI is poorly understood. A characteristic feature of oxPC(CD36) is an sn-2 acyl group that incorporates a terminal gamma-hydroxy (or oxo)-alpha,beta-unsaturated carbonyl. In the present study, a series of model oxidized phospholipids were designed, synthesized, and tested for their ability to serve as ligands for CD36 and SR-BI. We demonstrated that intact the sn-1 hydrophobic chain, the sn-3 hydrophilic phosphocholine or phosphatidic acid group, and the polar sn-2 tail are absolutely essential for high affinity binding. We further found that a terminal negatively charged carboxylate at the sn-2 position suffices to generate high binding affinity to class B scavenger receptors. In addition, factors such as polarity, rigidity, optimal chain length of sn-2, and sn-3 positions and negative charge at the sn-3 position of phospholipids further modulate the binding affinity. We conclude that all three positions of oxidized phospholipids are essential for the effective recognition by scavenger receptors class B. Furthermore, the structure of residues in these positions controls the affinity of the binding. The present studies suggest that, in addition to oxPC(CD36), other oxidized phospholipids observed in vivo may represent novel ligands for scavenger receptors class B.
Figures
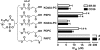
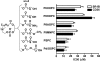

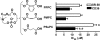

References
-
- Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Febbraio M., Hajjar D. P., Silverstein R. L., Hoff H. F., Salomon R. G., Hazen S. L. (2002) J. Biol. Chem. 277, 38517–38523 - PubMed
-
- Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Gugiu B., Fox P. L., Hoff H. F., Salomon R. G., Hazen S. L. (2002) J. Biol. Chem. 277, 38503–38516 - PubMed
-
- Greenberg M. E., Li X. M., Gugiu B. G., Gu X., Qin J., Salomon R. G., Hazen S. L. (2008) J. Biol. Chem. 283, 2385–2396 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

