Modulation of the human glucuronosyltransferase UGT1A pathway by splice isoform polypeptides is mediated through protein-protein interactions
- PMID: 19996319
- PMCID: PMC2823500
- DOI: 10.1074/jbc.M109.083139
Modulation of the human glucuronosyltransferase UGT1A pathway by splice isoform polypeptides is mediated through protein-protein interactions
Abstract
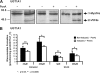
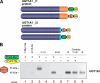
This study investigated the molecular mechanisms underlying the regulatory effect of the newly discovered 45-kDa enzymatically inactive UGT1A spliced polypeptides, named isoform i2, upon UGT1A-mediated glucuronidation. Initially, using an inducible system that mimics the relative abundance of isoforms 1 and 2 of UGT1A1 in human tissues, the rates of formation of glucuronides were significantly reduced. We then used a heterologous system constitutively expressing both isoforms i1 and i2 for an in-depth investigation of the presence of spliced i2 on glucuronidation kinetics. UGT1A1, UGT1A7, and UGT1A8 were selected as candidates for these studies. In all cases, co-expression of i1 and i2 in HEK293 cells leads to a significant reduction of the velocity of the glucuronidation reaction without affecting the affinity (K(m) (app)) for all substrates tested and the K(m) for the co-substrate, UDP-glucuronic acid. The data are consistent with a dominant-negative model of inhibition but do not sustain with an UGT1A_i2-mediated inhibition by competitive binding for substrate or the co-substrate. In contrast, the data from the co-immunoprecipitation experiments are indicative of the existence of a mixture homo-oligomeric (i1-i1 or i2-i2) and hetero-oligomeric (i1-i2) complexes in which the i2-i2 and i1-i2 subunits would be inactive. Thus, protein-protein interactions are likely responsible for the inhibition of active UGT1A_i1 by i2 spliced polypeptides. This new regulatory mechanism may alternatively modulate cellular response to endo/xeno stimulus.
Figures


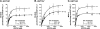


References
-
- Stamm S. (2008) J. Biol. Chem. 283, 1223–1227 - PubMed
-
- Girard H., Lévesque E., Bellemare J., Journault K., Caillier B., Guillemette C. (2007) Pharmacogenet. Genomics 17, 1077–1089 - PubMed
-
- Lévesque E., Girard H., Journault K., Lépine J., Guillemette C. (2007) Hepatology 45, 128–138 - PubMed
-
- Gong Q. H., Cho J. W., Huang T., Potter C., Gholami N., Basu N. K., Kubota S., Carvalho S., Pennington M. W., Owens I. S., Popescu N. C. (2001) Pharmacogenetics 11, 357–368 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

