Metabolic functions of atypical protein kinase C: "good" and "bad" as defined by nutritional status
- PMID: 19996389
- PMCID: PMC3774273
- DOI: 10.1152/ajpendo.00608.2009
Metabolic functions of atypical protein kinase C: "good" and "bad" as defined by nutritional status
Abstract
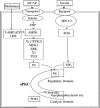
Atypical protein kinase C (aPKC) isoforms mediate insulin effects on glucose transport in muscle and adipose tissues and lipid synthesis in liver and support other metabolic processes, expression of enzymes needed for islet insulin secretion and hepatic glucose production/release, CNS appetite suppression, and inflammatory responses. In muscle, selective aPKC deficiency impairs glucose uptake and produces insulin resistance and hyperinsulinemia, which, by activating hepatic aPKC, provokes inordinate increases in lipid synthesis and produces typical "metabolic syndrome" features. In contrast, hepatic aPKC deficiency diminishes lipid synthesis and protects against metabolic syndrome features. Unfortunately, aPKC is deficient in muscle but paradoxically conserved in liver in obesity and type 2 diabetes mellitus; this combination is particularly problematic because it promotes lipid and carbohydrate abnormalities. Accordingly, metabolic effects of aPKCs can be "good" or "bad," depending upon nutritional status; thus, muscle glucose uptake, islet insulin secretion, hepatic glucose and lipid production/release, and adipose fat synthesis/storage would be important for survival during periods of limited food availability and therefore be "good." However, during times of food surfeit, excessive activation of hepatic aPKC, whether caused by overnutrition or impairments in extrahepatic effects of insulin, would lead to inordinate increases in hepatic lipid synthesis and metabolic syndrome features and therefore be "bad." In keeping with these ideas, the inhibition of hepatic aPKC markedly ameliorates lipid and carbohydrate abnormalities in experimental models of obesity and type 2 diabetes. We postulate that a similar approach may be useful for treating humans.
Figures
References
-
- Bandyopadhyay G, Kanoh Y, Sajan MP, Standaert ML, Farese RV. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-λ on insulin-stimulated glucose transport in L6 myotubes. Endocrinology 141: 4120–4127, 2000 - PubMed
-
- Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert ML, Quon MJ, Lea-Currie R, Sen A, Farese RV. PKC-zeta mediates insulin effects on glucose transport in cultured preadipocyte-derived human adipocytes. J Clin Endocrinol Metab 87: 716–723, 2002 - PubMed
-
- Bandyopadhyay G, Standaert ML, Galloway L, Moscat J, Farese RV. Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology 138: 4721–4731, 1997 - PubMed
-
- Bandyopadhyay G, Standaert ML, Kikkawa U, Ono Y, Moscat J, Farese RV. Effects of transiently expressed atypical (ζ, λ), conventional (α, β) and novel (δ, ε) protein kinase C isoforms on insulin-stimulated translocation of epitope-tagged GLUT4 glucose transporters in rat adipocytes. Specific interchangeable effects of protein kinase C-ζ and -λ. Biochem J 337: 461–470, 1999 - PMC - PubMed
-
- Bandyopadhyay G, Standaert ML, Zhao L, Yu B, Avignon A, Galloway L, Karnam P, Moscat J, Farese RV. Activation of PKC (α, β, and ζ) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-ζ in glucose transport. J Biol Chem 272: 2551–2558, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

