Collagenolytic activity is suppressed in organ-cultured human skin exposed to a gadolinium-based MRI contrast agent
- PMID: 19996758
- PMCID: PMC2819152
- DOI: 10.1097/RLI.0b013e3181bf95eb
Collagenolytic activity is suppressed in organ-cultured human skin exposed to a gadolinium-based MRI contrast agent
Abstract
Objective: Human skin produces increased amounts of matrix metalloproteinase-1 (MMP-1) when exposed in organ culture to Omniscan, one of the gadolinium-based MRI contrast agents (GBCA). MMP-1, by virtue of its ability to degrade structural collagen, contributes to collagen turnover in the skin. The objective of the present study was to determine whether collagenolytic activity was concomitantly up-regulated with increased enzyme.
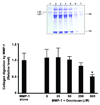
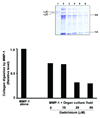
Materials and methods: Skin biopsies from normal volunteers were exposed in organ culture to Omniscan. Organ culture fluids obtained from control and treated skin were examined for ability to degrade type I collagen. The same culture fluids were examined for levels of MMP-1, tissue inhibitor of metalloproteinases-1 (TIMP-1), and complexes of MMP-1 and TIMP-1.
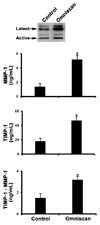
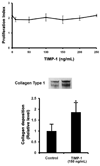
Results: Although MMP-1 was increased in culture fluid from Omniscan-treated skin, there was no increase in collagenolytic activity. In fact, collagenolytic activity declined. Increased production of TIMP-1 was also observed in Omniscan-treated skin, and the absolute amount of TIMP-1 was greater than the amount of MMP-1. Virtually all of the MMP-1 was present in MMP-1-TIMP-1 complexes, but the majority of TIMP-1 was not associated with MMP-1. When human dermal fibroblasts were exposed to TIMP-1 (up to 250 ng/mL), no increase in proliferation was observed, but an increase in collagen deposition into the cell layer was seen.
Conclusion: Gadolinium-based MRI contrast agent exposure has recently been linked to a fibrotic skin condition in patients with impaired kidney function. The mechanism is unknown. The increase in TIMP-1 production and concomitant reduction in collagenolytic activity demonstrated here could result in decreased collagen turnover and increased deposition of collagen in lesional skin.
Figures

Similar articles
-
Responses of human skin in organ culture and human skin fibroblasts to a gadolinium-based MRI contrast agent: comparison of skin from patients with end-stage renal disease and skin from healthy subjects.Invest Radiol. 2010 Nov;45(11):733-9. doi: 10.1097/RLI.0b013e3181e9436b. Invest Radiol. 2010. PMID: 20661146 Free PMC article.
-
Regulation of collagen turnover in human skin fibroblasts exposed to a gadolinium-based contrast agent.Invest Radiol. 2009 Aug;44(8):433-9. doi: 10.1097/RLI.0b013e3181a4d7e9. Invest Radiol. 2009. PMID: 19561517 Free PMC article.
-
Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin.Photochem Photobiol. 2003 Jul;78(1):43-8. doi: 10.1562/0031-8655(2003)078<0043:mmitmc>2.0.co;2. Photochem Photobiol. 2003. PMID: 12929747
-
Expression of 72-kDa gelatinase (MMP-2), collagenase (MMP-1), and tissue metalloproteinase inhibitor (TIMP) in primary pig skin fibroblast cultures derived from radiation-induced skin fibrosis.J Invest Dermatol. 1994 Jun;102(6):945-50. doi: 10.1111/1523-1747.ep12384118. J Invest Dermatol. 1994. PMID: 8006459
-
Effects of gadolinium-based magnetic resonance imaging contrast agents on human skin in organ culture and human skin fibroblasts.Invest Radiol. 2009 Feb;44(2):74-81. doi: 10.1097/RLI.0b013e31818f76b5. Invest Radiol. 2009. PMID: 19077912
Cited by
-
Understanding nephrogenic systemic fibrosis.Int J Nephrol. 2012;2012:912189. doi: 10.1155/2012/912189. Epub 2012 Nov 4. Int J Nephrol. 2012. PMID: 23193473 Free PMC article.
-
Fibroblast response to gadolinium: role for platelet-derived growth factor receptor.Invest Radiol. 2010 Dec;45(12):769-77. doi: 10.1097/RLI.0b013e3181e943d2. Invest Radiol. 2010. PMID: 20714270 Free PMC article.
-
Gadolinium-induced fibrosis is counter-regulated by CCN3 in human dermal fibroblasts: a model for potential treatment of nephrogenic systemic fibrosis.J Cell Commun Signal. 2012 Jun;6(2):97-105. doi: 10.1007/s12079-012-0164-4. Epub 2012 May 31. J Cell Commun Signal. 2012. PMID: 22648571 Free PMC article.
-
Responses of human skin in organ culture and human skin fibroblasts to a gadolinium-based MRI contrast agent: comparison of skin from patients with end-stage renal disease and skin from healthy subjects.Invest Radiol. 2010 Nov;45(11):733-9. doi: 10.1097/RLI.0b013e3181e9436b. Invest Radiol. 2010. PMID: 20661146 Free PMC article.
-
Fibroblast response to lanthanoid metal ion stimulation: potential contribution to fibrotic tissue injury.Biol Trace Elem Res. 2011 Dec;144(1-3):621-35. doi: 10.1007/s12011-011-9041-x. Epub 2011 Apr 12. Biol Trace Elem Res. 2011. PMID: 21484406 Free PMC article.
References
-
- Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000–1001. - PubMed
-
- Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563–572. - PubMed
-
- Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transpl. 2006;21:1104–1108. - PubMed
-
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359–2362. - PubMed
-
- Yerram P, Saab G, Karuparthi PR, et al. Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure-role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol. 2007;2:258–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

