Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision
- PMID: 19996955
- PMCID: PMC2907154
- DOI: 10.1097/ALN.0b013e3181c2952e
Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision
Abstract
Background: Guarding pain after rat plantar incision is similar to pain at rest in postoperative patients. Spontaneous activity (SA) in nociceptive pathways quite likely transmits such ongoing pain. This study examined the extent of tissue injury by incision on pain behaviors and nociceptor SA.
Methods: Rat pain behaviors were measured after a sham procedure, skin incision, or skin plus deep tissue incision. Separate groups of rats underwent in vivo single-fiber recording 1 day after a sham procedure, skin, or skin plus deep tissue incision or 7 days after skin plus deep tissue incision.
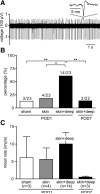
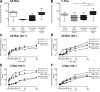
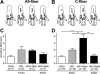
Results: Compared with the control procedure, skin incision induced moderate guarding on the day of incision only, whereas skin plus deep tissue incision caused guarding for 5 days. Mechanical and heat hyperalgesia were similar in both incised groups, except that mechanical hyperalgesia lasted longer after skin plus deep tissue incision. On Postoperative Day 1, skin incision (18.2%) produced a similar prevalence of SA in nociceptors as in controls (13.0%), whereas skin plus deep tissue incision generated a greater prevalence of SA (61.0%); SA rate also tended to be greater (6.1 vs. 10.0 imp/s) after skin plus deep tissue incision. Seven days after skin plus deep tissue incision, the SA prevalence was similar (13.6%) as in controls.
Conclusions: These data demonstrated that incised deep tissue rather than skin had a central role in the genesis of guarding behavior and nociceptor SA. Understanding the responses of deep tissue to incision and the mechanisms for deep tissue pain will improve postoperative pain management.
Figures





References
-
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–43. - PubMed
-
- Hamalainen MM, Gebhart GF, Brennan TJ. Acute effect of an incision on mechanosensitive afferents in the plantar rat hindpaw. J Neurophysiol. 2002;87:712–20. - PubMed
-
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. - PubMed
-
- Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North America. 2005;23:1–20. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

