Ethical considerations in the collection of genetic data from critically ill patients: what do published studies reveal about potential directions for empirical ethics research?
- PMID: 19997084
- PMCID: PMC2860600
- DOI: 10.1038/tpj.2009.61
Ethical considerations in the collection of genetic data from critically ill patients: what do published studies reveal about potential directions for empirical ethics research?
Abstract
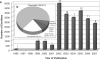
Critical illness trials involving genetic data collection are increasingly commonplace and pose challenges not encountered in less acute settings, related in part to the precipitous, severe and incapacitating nature of the diseases involved. We performed a systematic literature review to understand the nature of such studies conducted to date, and to consider, from an ethical perspective, potential barriers to future investigations. We identified 79 trials enrolling 24 499 subjects. Median (interquartile range) number of participants per study was 263 (116.75-430.75). Of these individuals, 16 269 (66.4%) were Caucasian, 1327 (5.4%) were African American, 1707 (7.0%) were Asian Pacific Islanders and 139 (0.6%) were Latino. For 5020 participants (20.5%), ethnicity was not reported. Forty-eight studies (60.8%) recruited subjects from single centers and all studies examined a relatively small number of genetic markers. Technological advances have rendered it feasible to conduct clinical studies using high-density genome-wide scanning. It will be necessary for future critical illness trials using these approaches to be of greater scope and complexity than those so far reported. Empirical research into issues related to greater ethnic inclusivity, accuracy of substituted judgment and specimen stewardship may be essential for enabling the conduct of such trials.
Figures
Similar articles
-
Racial Disparities in Sepsis-Related In-Hospital Mortality: Using a Broad Case Capture Method and Multivariate Controls for Clinical and Hospital Variables, 2004-2013.Crit Care Med. 2017 Dec;45(12):e1209-e1217. doi: 10.1097/CCM.0000000000002699. Crit Care Med. 2017. PMID: 28906287
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Ethical issues in neonatal research involving human subjects.Semin Perinatol. 2016 Jun;40(4):247-53. doi: 10.1053/j.semperi.2015.12.014. Epub 2016 Jan 21. Semin Perinatol. 2016. PMID: 26804381 Review.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Ethical issues in pragmatic randomized controlled trials: a review of the recent literature identifies gaps in ethical argumentation.BMC Med Ethics. 2018 Feb 27;19(1):14. doi: 10.1186/s12910-018-0253-x. BMC Med Ethics. 2018. PMID: 29482537 Free PMC article. Review.
Cited by
-
Ethical Aspects of Personalized Research and Management of Systemic Inflammatory Response Syndrome (SIRS) in Children.Int J Environ Res Public Health. 2022 Dec 28;20(1):470. doi: 10.3390/ijerph20010470. Int J Environ Res Public Health. 2022. PMID: 36612792 Free PMC article.
-
Research Ethics Challenges, Controversies and Difficulties in Intensive Care Units-A Systematic Review of Theoretical Concepts.Nurs Rep. 2025 May 7;15(5):164. doi: 10.3390/nursrep15050164. Nurs Rep. 2025. PMID: 40423198 Free PMC article. Review.
-
Surrogate receptivity to participation in critical illness genetic research: aligning research oversight and stakeholder concerns.Chest. 2015 Apr;147(4):979-988. doi: 10.1378/chest.14-0797. Chest. 2015. PMID: 25340645 Free PMC article.
-
Considerations in the construction of an instrument to assess attitudes regarding critical illness gene variation research.J Empir Res Hum Res Ethics. 2012 Feb;7(1):58-70. doi: 10.1525/jer.2012.7.1.58. J Empir Res Hum Res Ethics. 2012. PMID: 22378135 Free PMC article.
-
Tumor necrosis factor -308 polymorphism (rs1800629) is associated with mortality and ventilator duration in 1057 Caucasian patients.Cytokine. 2012 Oct;60(1):249-56. doi: 10.1016/j.cyto.2012.06.016. Epub 2012 Jun 28. Cytokine. 2012. PMID: 22749237 Free PMC article.
References
-
- Collins FS. Shattuck lecture—medical and societal consequences of the human genome project. N Engl J Med. 1999;342:28–37. - PubMed
-
- Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–491. - PubMed
-
- Evans WE, McLeod HL. Pharmacogenomics—drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–549. - PubMed
-
- Freeman BD, McLeod HL. Challenges of implementing pharmacogenetics in the critical care environment. Nat Rev Drug Discov. 2004;3:88–93. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

