Retinal imaging with polarization-sensitive optical coherence tomography and adaptive optics
- PMID: 19997405
- PMCID: PMC3113602
- DOI: 10.1364/OE.17.021634
Retinal imaging with polarization-sensitive optical coherence tomography and adaptive optics
Abstract
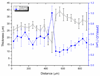
Various layers of the retina are well known to alter the polarization state of light. Such changes in polarization may be a sensitive indicator of tissue structure and function, and as such have gained increased clinical attention. Here we demonstrate a polarization-sensitive optical coherence tomography (PS-OCT) system that incorporates adaptive optics (AO) in the sample arm and a single line scan camera in the detection arm. We quantify the benefit of AO for PS-OCT in terms of signal-to-noise, lateral resolution, and speckle size. Double pass phase retardation per unit depth values ranging from 0.25 degrees/microm to 0.65 degrees/microm were found in the birefringent nerve fiber layer at 6 degrees eccentricity, superior to the fovea, with the highest values being noticeably higher than previously reported with PS-OCT around the optic nerve head. Moreover, fast axis orientation and degree of polarization uniformity measurements made with AO-PS-OCT demonstrate polarization scrambling in the retinal pigment epithelium at the highest resolution reported to date.
Figures






References
-
- Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch. Ophthalmol. 1982;100(1):135–146. - PubMed
-
- Bouma BE, Tearney GJ, editors. Handbook of Optical Coherence Tomography. New York: Marcel Dekker, Inc.; 2002.
-
- Medeiros FA, Zangwill LM, Bowd C, Weinreb RN. Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and stratus OCT optical coherence tomograph for the detection of glaucoma. Arch. Ophthalmol. 2004;122(6):827–837. - PubMed
-
- Wojtkowski M, Leitgeb R, Kowalczyk A, Bajraszewski T, Fercher AF. In vivo human retinal imaging by Fourier domain optical coherence tomography. J. Biomed. Opt. 2002;7(3):457–463. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

