High expression of CD244 and SAP regulated CD8 T cell responses of patients with HTLV-I associated neurologic disease
- PMID: 19997502
- PMCID: PMC2779586
- DOI: 10.1371/journal.ppat.1000682
High expression of CD244 and SAP regulated CD8 T cell responses of patients with HTLV-I associated neurologic disease
Abstract
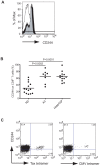
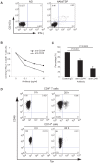
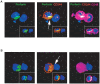
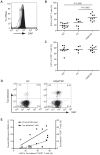
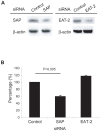
HTLV-I-specific CD8(+) T cells have been characterized with high frequencies in peripheral blood and cerebrospinal fluid and production of proinflammatory cytokines, which contribute to central nervous system inflammation in HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP). However, little is known about the differences in CD8(+) T cell activation status between asymptomatic carrier (ACs) and patients with HAM/TSP. The expression of CD244, a signaling lymphocyte activation molecule (SLAM) family receptor, was significantly higher on CD8(+) T cells in HTLV-I-infected patients, both ACs and patients with HAM/TSP, than those on healthy normal donors (NDs). Blockade of CD244 inhibited degranulation and IFN-gamma production in CD8(+) T cells of patients with HAM/TSP, suggesting that CD244 is associated with effector functions of CD8(+) T cells in patients with HAM/TSP. Moreover, SLAM-associated protein (SAP) was overexpressed in patients with HAM/TSP compared to ACs and NDs. SAP expression in Tax-specific CTLs was correlated in the HTLV-I proviral DNA loads and the frequency of the cells in HTLV-I-infected patients. SAP knockdown by siRNA also inhibited IFN-gamma production in CD8(+) T cells of patients with HAM/TSP. Thus, the CD244/SAP pathway was involved in the active regulation of CD8(+) T cells of patients with HAM/TSP, and may play roles in promoting inflammatory neurological disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, et al. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–281. - PubMed
-
- Gessain A, Barin F, Vernant JC, Gout O, Maurs L, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. - PubMed
-
- Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. - PubMed
-
- Umehara F, Izumo S, Nakagawa M, Ronquillo AT, Takahashi K, et al. Immunocytochemical analysis of the cellular infiltrate in the spinal cord lesions in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol. 1993;52:424–430. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

