Scrapie affects the maturation cycle and immune complex trapping by follicular dendritic cells in mice
- PMID: 19997557
- PMCID: PMC2785472
- DOI: 10.1371/journal.pone.0008186
Scrapie affects the maturation cycle and immune complex trapping by follicular dendritic cells in mice
Abstract
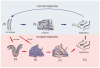
Transmissible spongiform encephalopathies (TSEs) or prion diseases are infectious neurological disorders of man and animals, characterised by abnormal disease-associated prion protein (PrP(d)) accumulations in the brain and lymphoreticular system (LRS). Prior to neuroinvasion, TSE agents often accumulate to high levels within the LRS, apparently without affecting immune function. However, our analysis of scrapie-affected sheep shows that PrP(d) accumulations within the LRS are associated with morphological changes to follicular dendritic cells (FDCs) and tingible body macrophages (TBMs). Here we examined FDCs and TBMs in the mesenteric lymph nodes (MLNs) of scrapie-affected mice by light and electron microscopy. In MLNs from uninfected mice, FDCs could be morphologically categorised into immature, mature and regressing forms. However, in scrapie-affected MLNs this maturation cycle was adversely affected. FDCs characteristically trap and retain immune complexes on their surfaces, which they display to B-lymphocytes. In scrapie-affected MLNs, some FDCs were found where areas of normal and abnormal immune complex retention occurred side by side. The latter co-localised with PrP(d) plasmalemmal accumulations. Our data suggest this previously unrecognised morphology represents the initial stage of an abnormal FDC maturation cycle. Alterations to the FDCs included PrP(d) accumulation, abnormal cell membrane ubiquitin and excess immunoglobulin accumulation. Regressing FDCs, in contrast, appeared to lose their membrane-attached PrP(d). Together, these data suggest that TSE infection adversely affects the maturation and regression cycle of FDCs, and that PrP(d) accumulation is causally linked to the abnormal pathology observed. We therefore support the hypothesis that TSEs cause an abnormality in immune function.
Conflict of interest statement
Figures
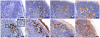




Similar articles
-
Scrapie-specific pathology of sheep lymphoid tissues.PLoS One. 2007 Dec 12;2(12):e1304. doi: 10.1371/journal.pone.0001304. PLoS One. 2007. PMID: 18074028 Free PMC article.
-
Murine scrapie infection causes an abnormal germinal centre reaction in the spleen.J Comp Pathol. 2004 Feb-Apr;130(2-3):181-94. doi: 10.1016/j.jcpa.2003.11.001. J Comp Pathol. 2004. PMID: 15003476
-
Sites of prion protein accumulation in scrapie-infected mouse spleen revealed by immuno-electron microscopy.J Pathol. 2000 Jul;191(3):323-32. doi: 10.1002/1096-9896(200007)191:3<323::AID-PATH629>3.0.CO;2-Z. J Pathol. 2000. PMID: 10878556
-
Follicular dendritic cells in scrapie pathogenesis.Arch Virol Suppl. 2000;(16):13-21. doi: 10.1007/978-3-7091-6308-5_2. Arch Virol Suppl. 2000. PMID: 11214915 Review.
-
Follicular dendritic cells as targets for intervention in transmissible spongiform encephalopathies.Semin Immunol. 2002 Aug;14(4):285-93. doi: 10.1016/s1044-5323(02)00061-1. Semin Immunol. 2002. PMID: 12163304 Review.
Cited by
-
Membrane toxicity of abnormal prion protein in adrenal chromaffin cells of scrapie infected sheep.PLoS One. 2013;8(3):e58620. doi: 10.1371/journal.pone.0058620. Epub 2013 Mar 4. PLoS One. 2013. PMID: 23469286 Free PMC article.
-
Altered lymphocyte proliferation and innate immune function in scrapie 139A- and ME7-infected mice.Viral Immunol. 2013 Jun;26(3):192-200. doi: 10.1089/vim.2012.0091. Epub 2013 May 8. Viral Immunol. 2013. PMID: 23656168 Free PMC article.
-
How do PrPSc Prions Spread between Host Species, and within Hosts?Pathogens. 2017 Nov 24;6(4):60. doi: 10.3390/pathogens6040060. Pathogens. 2017. PMID: 29186791 Free PMC article. Review.
-
Follicular dendritic cell-specific prion protein (PrP) expression alone is sufficient to sustain prion infection in the spleen.PLoS Pathog. 2011 Dec;7(12):e1002402. doi: 10.1371/journal.ppat.1002402. Epub 2011 Dec 1. PLoS Pathog. 2011. PMID: 22144895 Free PMC article.
-
Prion pathogenesis and secondary lymphoid organs (SLO): tracking the SLO spread of prions to the brain.Prion. 2012 Sep-Oct;6(4):322-33. doi: 10.4161/pri.20676. Epub 2012 Aug 16. Prion. 2012. PMID: 22895090 Free PMC article.
References
-
- Diringer H, Gelderblom H, Hilmert H, Ozel M, Edelbluth C. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983;306:476–478. - PubMed
-
- Manson J, McBride P, Hope J. Expression of the PrP Gene in the Brain of Sinc Congenic Mice and its Relationship to the Development of Scrapie. Neurodegeneration. 1992;1:45–52.
-
- Ford MJ, Burton LJ, Li H, Graham CH, Frobert Y, et al. A marked disparity between the expression of prion protein and its message by neurones of the CNS. Neuroscience. 2002;111:533–551. - PubMed
-
- Oesch B, Westaway D, Walchi M, McKinley MP, Kent SBH, et al. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985;40:735–746. - PubMed
-
- Fraser H, Dickinson AG. Pathogenesis of Scrapie in the Mouse: the Role of the Spleen. Nature. 1970;226:462–463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/D00831X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/A/00001660/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/A/00001659/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D00831X/3/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D00831X/2/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Research Materials

