Significance of initial blood pressure and comorbidity for the efficacy of a fixed combination of an angiotensin receptor blocker and hydrochlorothiazide in clinical practice
- PMID: 19997579
- PMCID: PMC2788603
- DOI: 10.2147/vhrm.s7756
Significance of initial blood pressure and comorbidity for the efficacy of a fixed combination of an angiotensin receptor blocker and hydrochlorothiazide in clinical practice
Abstract
Background: Two-thirds of all patients with arterial hypertension need drug combinations to achieve blood pressure (BP) goals. Fixed combinations have high efficacy and result in high patient compliance. 300 mg irbesartan plus 25 mg hydrochlorothiazide (HCTZ) has been investigated only in clinical trials but not in daily practice.
Methods: A multicenter, noninterventional, noncontrolled observational study with 8123 patients seen by 1604 physicians in daily practice. BP reduction (office measurements), co-morbid disease and tolerability were documented over a 6-month observational period.
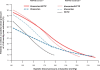
Results: At mean baseline BP of 161 +/- 15/94 +/- 10 mmHg, administering of fixed combination resulted in a substantial BP reduction averaging 28 +/- 15/14 +/- 10 mmHg (P < 0.001). Decrease of systolic BP ran parallel with increasing systolic baseline BP (Spearman's Rho -0.731; P < 0.0001; diastolic BP vs diastolic baseline BP Rho 0.740; P < 0.0001), independent from patient characteristics (age, obesity, diabetes or nephropathy) but enhanced with short history of hypertension (P < 0.0001 vs long history), prior beta blockers (P = 0.001 vs prior angiotensin receptor blockers [ARBs]), prior calcium channel blockers (P = 0.046 vs prior ARBs) and no prior medication (P = 0.012 vs prior ARBs). High compliance (>98%) and low incidence of adverse events (0.66%) were documented.
Conclusions: The fixed combination of 300 mg irbesartan with 25 mg HCTZ was efficacious and tolerable in an unselected patient population in primary care.
Keywords: combination therapy; diuretics; hypertension; irbesartan; noninterventional study; obesity.
Figures



Similar articles
-
Fixed combination of irbesartan and hydrochlorothiazide in the management of hypertension.Vasc Health Risk Manag. 2009;5(1):213-24. doi: 10.2147/vhrm.s3302. Epub 2009 Apr 8. Vasc Health Risk Manag. 2009. PMID: 19436667 Free PMC article. Review.
-
Irbesartan/hydrochlorothiazide for the treatment of isolated systolic hypertension: a subgroup analysis of the INCLUSIVE trial.J Natl Med Assoc. 2009 Apr;101(4):300-7. doi: 10.1016/s0027-9684(15)30876-2. J Natl Med Assoc. 2009. PMID: 19397219 Clinical Trial.
-
Management of hypertension with fixed dose combinations of candesartan cilexetil and hydrochlorothiazide: patient perspectives and clinical utility.Vasc Health Risk Manag. 2009;5:1043-58. doi: 10.2147/vhrm.s5549. Epub 2009 Dec 29. Vasc Health Risk Manag. 2009. PMID: 20057897 Free PMC article. Review.
-
A home blood pressure monitoring study comparing the antihypertensive efficacy of two angiotensin II receptor antagonist fixed combinations.Am J Hypertens. 2005 Nov;18(11):1482-8. doi: 10.1016/j.amjhyper.2005.06.009. Am J Hypertens. 2005. PMID: 16280286 Clinical Trial.
-
Safety and effectiveness of a fixed-dose combination of olmesartan, amlodipine, and hydrochlorothiazide in clinical practice.Vasc Health Risk Manag. 2014 Dec 17;11:1-8. doi: 10.2147/VHRM.S75380. eCollection 2015. Vasc Health Risk Manag. 2014. PMID: 25565857 Free PMC article.
Cited by
-
First Line CombinAtion Therapy in the Treatment of Stage II and III Hypertension (FLASH).Front Cardiovasc Med. 2020 Mar 30;7:46. doi: 10.3389/fcvm.2020.00046. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 32292790 Free PMC article.
-
Endothelial function, blood pressure control, and risk modification: impact of irbesartan alone or in combination.Integr Blood Press Control. 2010;3:21-30. doi: 10.2147/ibpc.s6081. Epub 2010 May 19. Integr Blood Press Control. 2010. PMID: 21949618 Free PMC article.
References
-
- Wolf-Maier K, Cooper RS, Banegas JR, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289(18):2363–2369. - PubMed
-
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22(1):11–19. - PubMed
-
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. - PubMed
-
- Leitlinien zur Behandlung der arteriellen Hypertonie 2008. Deutsche Hochdruckliga, 2008.
-
- Mancia G, De Backer G, Dominiczak A, et al. Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25(6):1105–1187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

