Therapeutic and adverse effects of a non-steroidal glucocorticoid receptor ligand in a mouse model of multiple sclerosis
- PMID: 19997594
- PMCID: PMC2781169
- DOI: 10.1371/journal.pone.0008202
Therapeutic and adverse effects of a non-steroidal glucocorticoid receptor ligand in a mouse model of multiple sclerosis
Abstract
Background: Dissociating glucocorticoid receptor (GR) ligands hold great promise for treating inflammatory disorders since it is assumed that they exert beneficial activities mediated by transrepression but avoid adverse effects of GR action requiring transactivation. Here we challenged this paradigm by investigating 2-(4-acetoxyphenyl)-2-chloro-N-methyl-ethylammonium chloride (CpdA), a dissociating non-steroidal GR ligand, in the context of experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS).
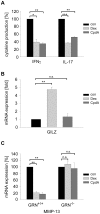
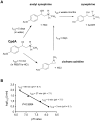
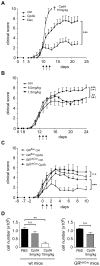
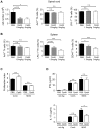
Methodology/principal findings: CpdA inhibited pro-inflammatory mediators in myelin-specific T cells and fibroblasts in a GR-dependent manner while gene activation was abolished. However, it also induced massive apoptosis in various cell types even in the absence of the GR by engaging a Bcl-2- and caspase-dependent pathway. (1)H NMR spectroscopy corroborated these findings by revealing that CpdA dissolved in buffered solutions rapidly decomposes into aziridine intermediates known to act as alkylating pro-apoptotic agents. Importantly, the dichotomy of CpdA action also became evident in vivo. Administration of high-dose CpdA to mice was lethal while treatment of EAE with low to intermediate amounts of CpdA dissolved in water significantly ameliorated the disease. The beneficial effect of CpdA required expression of the GR in T cells and was achieved by down regulating LFA-1 and CD44 on peripheral Th cells and by repressing IL-17 production.
Conclusions/significance: CpdA has significant therapeutic potential although adverse effects severely compromise its application in vivo. Hence, non-steroidal GR ligands require careful analysis prior to their translation into new therapeutic concepts.
Conflict of interest statement
Figures


References
-
- Lühder F, Reichardt HM. Traditional concepts and future avenues of glucocorticoid action in experimental autoimmune encephalomyelitis and multiple sclerosis therapy. Crit Rev Immunol. 2009;29:255–273. - PubMed
-
- Kirwan J, Power L. Glucocorticoids: action and new therapeutic insights in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:233–237. - PubMed
-
- Kleiman A, Tuckermann JP. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: lessons from conditional knockout mice. Mol Cell Endocrinol. 2007;275:98–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

