Reactive astrocytes in glial scar attract olfactory ensheathing cells migration by secreted TNF-alpha in spinal cord lesion of rat
- PMID: 19997621
- PMCID: PMC2780297
- DOI: 10.1371/journal.pone.0008141
Reactive astrocytes in glial scar attract olfactory ensheathing cells migration by secreted TNF-alpha in spinal cord lesion of rat
Abstract
Background: After spinal cord injury (SCI), the formation of glial scar contributes to the failure of injured adult axons to regenerate past the lesion. Increasing evidence indicates that olfactory ensheathing cells (OECs) implanted into spinal cord are found to migrate into the lesion site and induce axons regeneration beyond glial scar and resumption of functions. However, little is known about the mechanisms of OECs migrating from injection site to glial scar/lesion site.
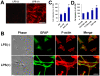
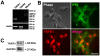
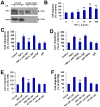
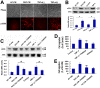
Methods and findings: In the present study, we identified a link between OECs migration and reactive astrocytes in glial scar that was mediated by the tumor necrosis factor-alpha (TNF-alpha). Initially, the Boyden chamber migration assay showed that both glial scar tissue and reactive astrocyte-conditioned medium promoted OECs migration in vitro. Reactive astrocyte-derived TNF-alpha and its type 1 receptor TNFR1 expressed on OECs were identified to be responsible for the promoting effect on OECs migration. TNF-alpha-induced OECs migration was demonstrated depending on activation of the extracellular signal-regulated kinase (ERK) signaling cascades. Furthermore, TNF-alpha secreted by reactive astrocytes in glial scar was also showed to attract OECs migration in a spinal cord hemisection injury model of rat.
Conclusions: These findings showed that TNF-alpha was released by reactive astrocytes in glial scar and attracted OECs migration by interacting with TNFR1 expressed on OECs via regulation of ERK signaling. This migration-attracting effect of reactive astrocytes on OECs may suggest a mechanism for guiding OECs migration into glial scar, which is crucial for OECs-mediated axons regrowth beyond the spinal cord lesion site.
Conflict of interest statement
Figures



References
-
- Cervos NJ, Lafuente JV. Traumatic brain injuries: structural changes. J Neurol Sci. 1991;103(Suppl):S3–14. - PubMed
-
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. - PubMed
-
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, et al. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. - PubMed
-
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

