Central role of the Holliday junction helicase RuvAB in vlsE recombination and infectivity of Borrelia burgdorferi
- PMID: 19997622
- PMCID: PMC2780311
- DOI: 10.1371/journal.ppat.1000679
Central role of the Holliday junction helicase RuvAB in vlsE recombination and infectivity of Borrelia burgdorferi
Abstract
Antigenic variation plays a vital role in the pathogenesis of many infectious bacteria and protozoa including Borrelia burgdorferi, the causative agent of Lyme disease. VlsE, a 35 kDa surface-exposed lipoprotein, undergoes antigenic variation during B. burgdorferi infection of mammalian hosts, and is believed to be a critical mechanism by which the spirochetes evade immune clearance. Random, segmental recombination between the expressed vlsE gene and adjacent vls silent cassettes generates a large number of different VlsE variants within the infected host. Although the occurrence and importance of vlsE sequence variation is well established, little is known about the biological mechanism of vlsE recombination. To identify factors important in antigenic variation and vlsE recombination, we screened transposon mutants of genes known to be involved in DNA recombination and repair for their effects on infectivity and vlsE recombination. Several mutants, including those in BB0023 (ruvA), BB0022 (ruvB), BB0797 (mutS), and BB0098 (mutS-II), showed reduced infectivity in immunocompetent C3H/HeN mice. Mutants in ruvA and ruvB exhibited greatly reduced rates of vlsE recombination in C3H/HeN mice, as determined by restriction fragment polymorphism (RFLP) screening and DNA sequence analysis. In severe combined immunodeficiency (C3H/scid) mice, the ruvA mutant retained full infectivity; however, all recovered clones retained the 'parental' vlsE sequence, consistent with low rates of vlsE recombination. These results suggest that the reduced infectivity of ruvA and ruvB mutants is the result of ineffective vlsE recombination and underscores the important role that vlsE recombination plays in immune evasion. Based on functional studies in other organisms, the RuvAB complex of B. burgdorferi may promote branch migration of Holliday junctions during vlsE recombination. Our findings are consistent with those in the accompanying article by Dresser et al., and together these studies provide the first examples of trans-acting factors involved in vlsE recombination.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


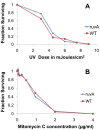
Similar articles
-
Investigation of the genes involved in antigenic switching at the vlsE locus in Borrelia burgdorferi: an essential role for the RuvAB branch migrase.PLoS Pathog. 2009 Dec;5(12):e1000680. doi: 10.1371/journal.ppat.1000680. Epub 2009 Dec 4. PLoS Pathog. 2009. PMID: 19997508 Free PMC article.
-
Effects of vlsE complementation on the infectivity of Borrelia burgdorferi lacking the linear plasmid lp28-1.Infect Immun. 2004 Nov;72(11):6577-85. doi: 10.1128/IAI.72.11.6577-6585.2004. Infect Immun. 2004. PMID: 15501789 Free PMC article.
-
Detailed analysis of sequence changes occurring during vlsE antigenic variation in the mouse model of Borrelia burgdorferi infection.PLoS Pathog. 2009 Feb;5(2):e1000293. doi: 10.1371/journal.ppat.1000293. Epub 2009 Feb 13. PLoS Pathog. 2009. PMID: 19214205 Free PMC article.
-
vls Antigenic Variation Systems of Lyme Disease Borrelia: Eluding Host Immunity through both Random, Segmental Gene Conversion and Framework Heterogeneity.Microbiol Spectr. 2014 Dec;2(6):10.1128/microbiolspec.MDNA3-0038-2014. doi: 10.1128/microbiolspec.MDNA3-0038-2014. Microbiol Spectr. 2014. PMID: 26104445 Free PMC article. Review.
-
Changing of the guard: How the Lyme disease spirochete subverts the host immune response.J Biol Chem. 2020 Jan 10;295(2):301-313. doi: 10.1074/jbc.REV119.008583. Epub 2019 Nov 21. J Biol Chem. 2020. PMID: 31753921 Free PMC article. Review.
Cited by
-
The telomere resolvase of the Lyme disease spirochete, Borrelia burgdorferi, promotes DNA single-strand annealing and strand exchange.Nucleic Acids Res. 2013 Dec;41(22):10438-48. doi: 10.1093/nar/gkt832. Epub 2013 Sep 17. Nucleic Acids Res. 2013. PMID: 24049070 Free PMC article.
-
Functional analysis of DNA replication fork reversal catalyzed by Mycobacterium tuberculosis RuvAB proteins.J Biol Chem. 2012 Jan 6;287(2):1345-60. doi: 10.1074/jbc.M111.304741. Epub 2011 Nov 17. J Biol Chem. 2012. PMID: 22094465 Free PMC article.
-
Focusing homologous recombination: pilin antigenic variation in the pathogenic Neisseria.Mol Microbiol. 2011 Sep;81(5):1136-43. doi: 10.1111/j.1365-2958.2011.07773.x. Epub 2011 Aug 4. Mol Microbiol. 2011. PMID: 21812841 Free PMC article. Review.
-
YebC regulates variable surface antigen VlsE expression and is required for host immune evasion in Borrelia burgdorferi.PLoS Pathog. 2020 Oct 13;16(10):e1008953. doi: 10.1371/journal.ppat.1008953. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33048986 Free PMC article.
-
Genetics of Borrelia burgdorferi.Annu Rev Genet. 2012;46:515-36. doi: 10.1146/annurev-genet-011112-112140. Epub 2012 Sep 4. Annu Rev Genet. 2012. PMID: 22974303 Free PMC article. Review.
References
-
- Lane RS, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. - PubMed
-
- Wisniewski-Dye F, Vial L. Phase and antigenic variation mediated by genome modifications. Antonie Van Leeuwenhoek. 2008;94:493–515. - PubMed
-
- Wang D, Botkin DJ, Norris SJ. Characterization of the vls antigenic variation loci of the Lyme disease spirochaetes Borrelia garinii Ip90 and Borrelia afzelii ACAI. Mol Microbiol. 2003;47:1407–1417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

