High antibody titer against apical membrane antigen-1 is required to protect against malaria in the Aotus model
- PMID: 19997632
- PMCID: PMC2780715
- DOI: 10.1371/journal.pone.0008138
High antibody titer against apical membrane antigen-1 is required to protect against malaria in the Aotus model
Abstract
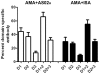
A Plasmodium falciparum 3D7 strain Apical Membrane Antigen-1 (AMA1) vaccine, formulated with AS02(A) adjuvant, slowed parasite growth in a recent Phase 1/2a trial, however sterile protection was not observed. We tested this AS02(A), and a Montanide ISA720 (ISA) formulation of 3D7 AMA1 in Aotus monkeys. The 3D7 parasite does not invade Aotus erythrocytes, hence two heterologous strains, FCH/4 and FVO, were used for challenge, FCH/4 AMA1 being more homologous to 3D7 than FVO AMA1. Following three vaccinations, the monkeys were challenged with 50,000 FCH/4 or 10,000 FVO parasites. Three of the six animals in the AMA+ISA group were protected against FCH/4 challenge. One monkey did not become parasitemic, another showed only a short period of low level parasitemia that self-cured, and a third animal showed a delay before exhibiting its parasitemic phase. This is the first protection shown in primates with a recombinant P. falciparum AMA1 without formulation in Freund's complete adjuvant. No animals in the AMA+AS02(A) group were protected, but this group exhibited a trend towards reduced growth rate. A second group of monkeys vaccinated with AMA+ISA vaccine was not protected against FVO challenge, suggesting strain-specificity of AMA1-based protection. Protection against FCH/4 strain correlated with the quantity of induced antibodies, as the protected animals were the only ones to have in vitro parasite growth inhibitory activity of >70% at 1:10 serum dilution; immuno-fluorescence titers >8,000; ELISA titers against full-length AMA1 >300,000 and ELISA titer against AMA1 domains1+2 >100,000. A negative correlation between log ELISA titer and day 11 cumulative parasitemia (Spearman rank r = -0.780, p value = 0.0001), further confirmed the relationship between antibody titer and protection. High titers of cross-strain inhibitory antibodies against AMA1 are therefore critical to confer solid protection, and the Aotus model can be used to down-select future AMA1 formulations, prior to advanced human trials.
Conflict of interest statement
Figures
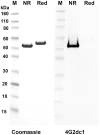
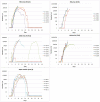


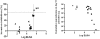
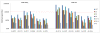
References
-
- Heppner DG, Jr., Kester KE, Ockenhouse CF, Tornieporth N, Ofori O, et al. Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine. 2005;23:2243–2250. - PubMed
-
- Bannister LH, Hopkins JM, Dluzewski AR, Margos G, Williams IT, et al. Plasmodium falciparum apical membrane antigen 1 (PfAMA-1) is translocated within micronemes along subpellicular microtubules during merozoite development. J Cell Sci. 2003;116:3825–3834. - PubMed
-
- Triglia T, Healer J, Caruana SR, Hodder AN, Anders RF, et al. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol Microbiol. 2000;38:706–718. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

